A system to enrich for primitive streak-derivatives, definitive endoderm and mesoderm, from pluripotent cells in culture
- PMID: 22701686
- PMCID: PMC3372479
- DOI: 10.1371/journal.pone.0038645
A system to enrich for primitive streak-derivatives, definitive endoderm and mesoderm, from pluripotent cells in culture
Abstract
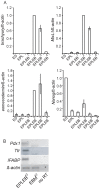
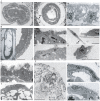
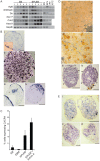
Two lineages of endoderm develop during mammalian embryogenesis, the primitive endoderm in the pre-implantation blastocyst and the definitive endoderm at gastrulation. This complexity of endoderm cell populations is mirrored during pluripotent cell differentiation in vitro and has hindered the identification and purification of the definitive endoderm for use as a substrate for further differentiation. The aggregation and differentiation of early primitive ectoderm-like (EPL) cells, resulting in the formation of EPL-cell derived embryoid bodies (EPLEBs), is a model of gastrulation that progresses through the sequential formation of primitive streak-like intermediates to nascent mesoderm and more differentiated mesoderm populations. EPL cell-derived EBs have been further analysed for the formation of definitive endoderm by detailed morphological studies, gene expression and a protein uptake assay. In comparison to embryoid bodies derived from ES cells, which form primitive and definitive endoderm, the endoderm compartment of embryoid bodies formed from EPL cells was comprised almost exclusively of definitive endoderm. Definitive endoderm was defined as a population of squamous cells that expressed Sox17, CXCR4 and Trh, which formed without the prior formation of primitive endoderm and was unable to endocytose horseradish peroxidase from the medium. Definitive endoderm formed in EPLEBs provides a substrate for further differentiation into specific endoderm lineages; these lineages can be used as research tools for understanding the mechanisms controlling lineage establishment and the nature of the transient intermediates formed. The similarity between mouse EPL cells and human ES cells suggests EPLEBs can be used as a model system for the development of technologies to enrich for the formation of human ES cell-derived definitive endoderm in the future.
Conflict of interest statement
Figures

Similar articles
-
Endoderm complexity in the mouse gastrula is revealed through the expression of spink3.Biores Open Access. 2014 Jun 1;3(3):98-109. doi: 10.1089/biores.2014.0010. Biores Open Access. 2014. PMID: 24940561 Free PMC article.
-
Reversible programming of pluripotent cell differentiation.J Cell Sci. 2000 Feb;113 ( Pt 3):555-66. doi: 10.1242/jcs.113.3.555. J Cell Sci. 2000. PMID: 10639341
-
A requirement for FGF signalling in the formation of primitive streak-like intermediates from primitive ectoderm in culture.PLoS One. 2010 Sep 3;5(9):e12555. doi: 10.1371/journal.pone.0012555. PLoS One. 2010. PMID: 20838439 Free PMC article.
-
Differentiation of definitive endoderm from mouse embryonic stem cells.Results Probl Cell Differ. 2012;55:303-19. doi: 10.1007/978-3-642-30406-4_17. Results Probl Cell Differ. 2012. PMID: 22918814 Review.
-
A little winning streak: the reptilian-eye view of gastrulation in birds.Dev Growth Differ. 2013 Jan;55(1):52-9. doi: 10.1111/dgd.12014. Epub 2012 Nov 16. Dev Growth Differ. 2013. PMID: 23157408 Review.
Cited by
-
Endoderm complexity in the mouse gastrula is revealed through the expression of spink3.Biores Open Access. 2014 Jun 1;3(3):98-109. doi: 10.1089/biores.2014.0010. Biores Open Access. 2014. PMID: 24940561 Free PMC article.
-
Neural Commitment of Embryonic Stem Cells through the Formation of Embryoid Bodies (EBs).Malays J Med Sci. 2014 Sep-Oct;21(5):8-16. Malays J Med Sci. 2014. PMID: 25977628 Free PMC article.
-
Src Family Kinases and p38 Mitogen-Activated Protein Kinases Regulate Pluripotent Cell Differentiation in Culture.PLoS One. 2016 Oct 10;11(10):e0163244. doi: 10.1371/journal.pone.0163244. eCollection 2016. PLoS One. 2016. PMID: 27723793 Free PMC article.
References
-
- Chazaud C, Yamanaka Y, Pawson T, Rossant J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev Cell. 2006;10:615–624. - PubMed
-
- Hogan BL, Taylor A, Adamson E. Cell interactions modulate embryonal carcinoma cell differentiation into parietal or visceral endoderm. Nature. 1981;291:235–237. - PubMed
-
- Hogan BL, Tilly R. Cell interactions and endoderm differentiation in cultured mouse embryos. J Embryol Exp Morphol. 1981;62:379–394. - PubMed
-
- Tam PP, Beddington RS. Establishment and organization of germ layers in the gastrulating mouse embryo. Ciba Found Symp 165: 27–41; discussion 42–29. 1992. - PubMed
-
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, et al. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

