A single ligand is sufficient to activate EGFR dimers
- PMID: 22699492
- PMCID: PMC3390854
- DOI: 10.1073/pnas.1201114109
A single ligand is sufficient to activate EGFR dimers
Abstract
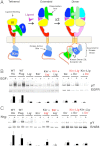
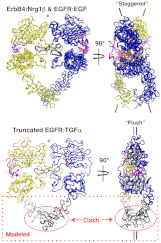
Crystal structures of human epidermal growth factor receptor (EGFR) with bound ligand revealed symmetric, doubly ligated receptor dimers thought to represent physiologically active states. Such complexes fail to rationalize negative cooperativity of epidermal growth factor (EGF) binding to EGFR and the behavior of the ligandless EGFR homolog ErbB2/HER2, however. We report cell-based assays that provide evidence for active, singly ligated dimers of human EGFR and its homolog, ErbB4/HER4. We also report crystal structures of the ErbB4/HER4 extracellular region complexed with its ligand Neuregulin-1β that resolve two types of ErbB dimer when compared to EGFR:Ligand complexes. One type resembles the recently reported asymmetric dimer of Drosophila EGFR with a single high-affinity ligand bound and provides a model for singly ligated human ErbB dimers. These results unify models of vertebrate and invertebrate EGFR/ErbB signaling, imply that the tethered conformation of unliganded ErbBs evolved to prevent crosstalk among ErbBs, and establish a molecular basis for both negative cooperativity of ligand binding to vertebrate ErbBs and the absence of active ErbB2/HER2 homodimers in normal conditions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
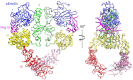

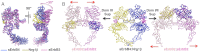
Similar articles
-
ErbB2 resembles an autoinhibited invertebrate epidermal growth factor receptor.Nature. 2009 Sep 10;461(7261):287-91. doi: 10.1038/nature08297. Epub 2009 Aug 30. Nature. 2009. PMID: 19718021 Free PMC article.
-
The extracellular region of ErbB4 adopts a tethered conformation in the absence of ligand.Proc Natl Acad Sci U S A. 2005 Oct 18;102(42):15024-9. doi: 10.1073/pnas.0507591102. Epub 2005 Oct 3. Proc Natl Acad Sci U S A. 2005. PMID: 16203964 Free PMC article.
-
The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors.Mol Cell. 2003 Feb;11(2):495-505. doi: 10.1016/s1097-2765(03)00048-0. Mol Cell. 2003. PMID: 12620236
-
ErbB Receptors and Cancer.Methods Mol Biol. 2017;1652:3-35. doi: 10.1007/978-1-4939-7219-7_1. Methods Mol Biol. 2017. PMID: 28791631 Review.
-
The ErbB/HER family of protein-tyrosine kinases and cancer.Pharmacol Res. 2014 Jan;79:34-74. doi: 10.1016/j.phrs.2013.11.002. Epub 2013 Nov 20. Pharmacol Res. 2014. PMID: 24269963 Review.
Cited by
-
An effective strategy for ligand-mediated pulldown of the HER2/HER3/NRG1β heterocomplex and cryo-EM structure determination at low sample concentrations.Methods Enzymol. 2022;667:633-662. doi: 10.1016/bs.mie.2022.03.049. Epub 2022 Apr 20. Methods Enzymol. 2022. PMID: 35525557 Free PMC article.
-
Mechanism of Allosteric Coupling into and through the Plasma Membrane by EGFR.Cell Chem Biol. 2018 Jul 19;25(7):857-870.e7. doi: 10.1016/j.chembiol.2018.04.005. Epub 2018 May 3. Cell Chem Biol. 2018. PMID: 29731426 Free PMC article.
-
Inhibitor-induced HER2-HER3 heterodimerisation promotes proliferation through a novel dimer interface.Elife. 2018 May 1;7:e32271. doi: 10.7554/eLife.32271. Elife. 2018. PMID: 29712619 Free PMC article.
-
Piecing it together: Unraveling the elusive structure-function relationship in single-pass membrane receptors.Biochim Biophys Acta Biomembr. 2017 Sep;1859(9 Pt A):1398-1416. doi: 10.1016/j.bbamem.2017.01.016. Epub 2017 Jan 12. Biochim Biophys Acta Biomembr. 2017. PMID: 28089689 Free PMC article. Review.
-
Profiling epidermal growth factor receptor and heregulin receptor 3 heteromerization using receptor tyrosine kinase heteromer investigation technology.PLoS One. 2013 May 20;8(5):e64672. doi: 10.1371/journal.pone.0064672. Print 2013. PLoS One. 2013. PMID: 23700486 Free PMC article.
References
-
- Burgess AW. EGFR family: Structure physiology signalling and therapeutic targets. Growth Factors. 2008;26:263–274. - PubMed
-
- Heldin CH. Dimerization of cell surface receptors in signal transduction. Cell. 1995;80:213–223. - PubMed
-
- Yarden Y, Schlessinger J. Epidermal growth factor induces rapid, reversible aggregation of the purified epidermal growth factor receptor. Biochemistry. 1987;26:1443–1451. - PubMed
-
- Burgess AW, et al. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell. 2003;12:541–552. - PubMed
-
- Chung I, et al. Spatial control of EGF receptor activation by reversible dimerization on living cells. Nature. 2010;464(7289):783–787. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

