FGF9 and FGF20 maintain the stemness of nephron progenitors in mice and man
- PMID: 22698282
- PMCID: PMC3376351
- DOI: 10.1016/j.devcel.2012.04.018
FGF9 and FGF20 maintain the stemness of nephron progenitors in mice and man
Abstract
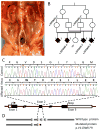
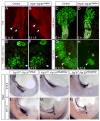
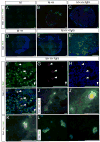
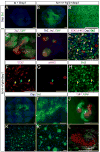
The identity of niche signals necessary to maintain embryonic nephron progenitors is unclear. Here we provide evidence that Fgf20 and Fgf9, expressed in the niche, and Fgf9, secreted from the adjacent ureteric bud, are necessary and sufficient to maintain progenitor stemness. Reduction in the level of these redundant ligands in the mouse led to premature progenitor differentiation within the niche. Loss of FGF20 in humans, or of both ligands in mice, resulted in kidney agenesis. Sufficiency was shown in vitro where Fgf20 or Fgf9 (alone or together with Bmp7) maintained isolated metanephric mesenchyme or sorted nephron progenitors that remained competent to differentiate in response to Wnt signals after 5 or 2 days in culture, respectively. These findings identify a long-sought-after critical component of the nephron stem cell niche and hold promise for long-term culture and utilization of these progenitors in vitro.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
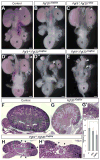
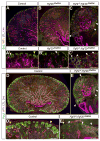
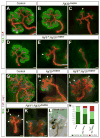
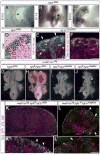
Comment in
-
How the developing mammalian kidney assembles its thousands of nephrons: Fgfs as stemness signals.Dev Cell. 2012 Jun 12;22(6):1125-6. doi: 10.1016/j.devcel.2012.05.016. Dev Cell. 2012. PMID: 22698278
Similar articles
-
Nephron Progenitor Maintenance Is Controlled through Fibroblast Growth Factors and Sprouty1 Interaction.J Am Soc Nephrol. 2020 Nov;31(11):2559-2572. doi: 10.1681/ASN.2020040401. Epub 2020 Aug 4. J Am Soc Nephrol. 2020. PMID: 32753399 Free PMC article.
-
[FGF9 and FGF20 maintain the stemness of nephron progenitors during kidney development].Med Sci (Paris). 2013 Mar;29(3):254-6. doi: 10.1051/medsci/2013293009. Epub 2013 Mar 27. Med Sci (Paris). 2013. PMID: 23544377 French. No abstract available.
-
Role of FGFRL1 and other FGF signaling proteins in early kidney development.Cell Mol Life Sci. 2013 Jul;70(14):2505-18. doi: 10.1007/s00018-012-1189-9. Epub 2012 Oct 31. Cell Mol Life Sci. 2013. PMID: 23112089 Free PMC article. Review.
-
Defining the signals that constitute the nephron progenitor niche.J Am Soc Nephrol. 2013 May;24(6):873-6. doi: 10.1681/ASN.2012090931. Epub 2013 Apr 11. J Am Soc Nephrol. 2013. PMID: 23578945
-
Nephron progenitors in the metanephric mesenchyme.Pediatr Nephrol. 2011 Sep;26(9):1463-7. doi: 10.1007/s00467-011-1806-0. Epub 2011 Feb 19. Pediatr Nephrol. 2011. PMID: 21336811 Review.
Cited by
-
Fgf9 regulates bone marrow mesenchymal stem cell fate and bone-fat balance in osteoporosis by PI3K/AKT/Hippo and MEK/ERK signaling.Int J Biol Sci. 2024 Jun 17;20(9):3461-3479. doi: 10.7150/ijbs.94863. eCollection 2024. Int J Biol Sci. 2024. PMID: 38993574 Free PMC article.
-
Nephron progenitors rhythmically alternate between renewal and differentiation phases that synchronize with kidney branching morphogenesis.bioRxiv [Preprint]. 2024 Jan 13:2023.11.21.568157. doi: 10.1101/2023.11.21.568157. bioRxiv. 2024. PMID: 38045273 Free PMC article. Preprint.
-
Kidney organoids: accurate models or fortunate accidents.Genes Dev. 2019 Oct 1;33(19-20):1319-1345. doi: 10.1101/gad.329573.119. Genes Dev. 2019. PMID: 31575677 Free PMC article. Review.
-
Fgf8 promotes survival of nephron progenitors by regulating BAX/BAK-mediated apoptosis.Differentiation. 2023 Mar-Apr;130:7-15. doi: 10.1016/j.diff.2022.12.001. Epub 2022 Dec 6. Differentiation. 2023. PMID: 36527791 Free PMC article.
-
Whole-Exome Sequencing Identifies Causative Mutations in Families with Congenital Anomalies of the Kidney and Urinary Tract.J Am Soc Nephrol. 2018 Sep;29(9):2348-2361. doi: 10.1681/ASN.2017121265. Epub 2018 Aug 24. J Am Soc Nephrol. 2018. PMID: 30143558 Free PMC article.
References
-
- Barak H, Boyle SC. Organ culture and immunostaining of mouse embryonic kidneys. Cold Spring Harb Protoc 2011. 2011 pdb prot5558. - PubMed
-
- Barasch J, Qiao J, McWilliams G, Chen D, Oliver JA, Herzlinger D. Ureteric bud cells secrete multiple factors, including bFGF, which rescue renal progenitors from apoptosis. Am J Physiol. 1997;273:F757–767. - PubMed
-
- Bates CM. Role of fibroblast growth factor receptor signaling in kidney development. Pediatr Nephrol 2011 - PubMed
-
- Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE. Human nephron number: implications for health and disease. Pediatr Nephrol. 2011;26:1529–1533. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

