Impairment of GABAB receptor dimer by endogenous 14-3-3ζ in chronic pain conditions
- PMID: 22692127
- PMCID: PMC3411072
- DOI: 10.1038/emboj.2012.161
Impairment of GABAB receptor dimer by endogenous 14-3-3ζ in chronic pain conditions
Abstract
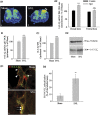
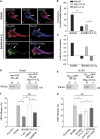
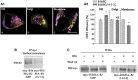
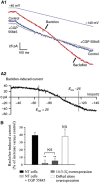
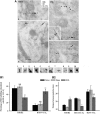
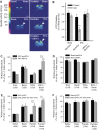
In the central nervous system, the inhibitory GABAB receptor is the archetype of heterodimeric G protein-coupled receptors (GPCRs). However, the regulation of GABAB dimerization, and more generally of GPCR oligomerization, remains largely unknown. We propose a novel mechanism for inhibition of GPCR activity through de-dimerization in pathological conditions. We show here that 14-3-3ζ, a GABAB1-binding protein, dissociates the GABAB heterodimer, resulting in the impairment of GABAB signalling in spinal neurons. In the dorsal spinal cord of neuropathic rats, 14-3-3ζ is overexpressed and weakens GABAB inhibition. Using anti-14-3-3ζ siRNA or competing peptides disrupts 14-3-3ζ/GABAB1 interaction and restores functional GABAB heterodimers in the dorsal horn. Importantly, both strategies greatly enhance the anti-nociceptive effect of intrathecal Baclofen in neuropathic rats. Taken together, our data provide the first example of endogenous regulation of a GPCR oligomeric state and demonstrate its functional impact on the pathophysiological process of neuropathic pain sensitization.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Comment in
-
Divorce of obligatory partners in pain: disruption of GABA(B) receptor heterodimers in neuralgia.EMBO J. 2012 Aug 1;31(15):3234-6. doi: 10.1038/emboj.2012.174. Epub 2012 Jun 26. EMBO J. 2012. PMID: 22735189 Free PMC article.
Similar articles
-
Spinal Inhibition of GABAB Receptors by the Extracellular Matrix Protein Fibulin-2 in Neuropathic Rats.Front Cell Neurosci. 2020 Jul 15;14:214. doi: 10.3389/fncel.2020.00214. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32765223 Free PMC article.
-
Relationship between the antinociceptive response to desipramine and changes in GABAB receptor function and subunit expression in the dorsal horn of the rat spinal cord.Biochem Pharmacol. 2004 Feb 15;67(4):743-9. doi: 10.1016/j.bcp.2003.10.008. Biochem Pharmacol. 2004. PMID: 14757174
-
Dissociation and trafficking of rat GABAB receptor heterodimer upon chronic capsaicin stimulation.Eur J Neurosci. 2007 Mar;25(5):1402-16. doi: 10.1111/j.1460-9568.2007.05398.x. Eur J Neurosci. 2007. PMID: 17425567
-
[GABA(B) receptors and sensitization to pain].J Soc Biol. 2009;203(1):87-97. doi: 10.1051/jbio:2009009. Epub 2009 Apr 10. J Soc Biol. 2009. PMID: 19358814 Review. French.
-
Structural biology of GABAB receptor.Neuropharmacology. 2018 Jul 1;136(Pt A):68-79. doi: 10.1016/j.neuropharm.2017.10.011. Epub 2017 Oct 12. Neuropharmacology. 2018. PMID: 29031577 Free PMC article. Review.
Cited by
-
MicroRNA-330 Directs Downregulation of the GABABR2 in the Pathogenesis of Pancreatic Cancer Pain.J Mol Neurosci. 2020 Oct;70(10):1541-1551. doi: 10.1007/s12031-020-01607-7. Epub 2020 Jul 4. J Mol Neurosci. 2020. PMID: 32621101
-
Calcium signalling through L-type calcium channels: role in pathophysiology of spinal nociceptive transmission.Br J Pharmacol. 2018 Jun;175(12):2362-2374. doi: 10.1111/bph.13747. Epub 2017 Mar 24. Br J Pharmacol. 2018. PMID: 28214378 Free PMC article. Review.
-
The Retinitis Pigmentosa-Linked Mutations in Transmembrane Helix 5 of Rhodopsin Disrupt Cellular Trafficking Regardless of Oligomerization State.Biochemistry. 2018 Sep 4;57(35):5188-5201. doi: 10.1021/acs.biochem.8b00403. Epub 2018 Aug 21. Biochemistry. 2018. PMID: 30085663 Free PMC article.
-
Modulating GPCR and 14-3-3 protein interactions: Prospects for CNS drug discovery.Drug Discov Today. 2023 Aug;28(8):103641. doi: 10.1016/j.drudis.2023.103641. Epub 2023 May 24. Drug Discov Today. 2023. PMID: 37236523 Free PMC article. Review.
-
Organization and functions of mGlu and GABAB receptor complexes.Nature. 2016 Dec 1;540(7631):60-68. doi: 10.1038/nature20566. Nature. 2016. PMID: 27905440 Review.
References
-
- Attal N, Cruccu G, Haanpaa M, Hansson P, Jensen TS, Nurmikko T, Sampaio C, Sindrup S, Wiffen P (2006) EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol 13: 1153–1169 - PubMed
-
- Bardoni R, Ghirri A, Salio C, Prandini M, Merighi A (2007) BDNF-mediated modulation of GABA and glycine release in dorsal horn lamina II from postnatal rats. Dev Neurobiol 67: 960–975 - PubMed
-
- Benke D, Honer M, Michel C, Bettler B, Mohler H (1999) gamma-aminobutyric acid type B receptor splice variant proteins GBR1a and GBR1b are both associated with GBR2 in situ and display differential regional and subcellular distribution. J Biol Chem 274: 27323–27330 - PubMed
-
- Berg D, Holzmann C, Riess O (2003) 14-3-3 proteins in the nervous system. Nat Rev Neurosci 4: 752–762 - PubMed
-
- Bouvier M (2001) Oligomerization of G-protein-coupled transmitter receptors. Nat Rev Neurosci 2: 274–286 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

