Epithelial-mesenchymal transition increases tumor sensitivity to COX-2 inhibition by apricoxib
- PMID: 22678114
- PMCID: PMC3514897
- DOI: 10.1093/carcin/bgs195
Epithelial-mesenchymal transition increases tumor sensitivity to COX-2 inhibition by apricoxib
Abstract
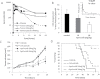
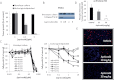
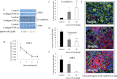
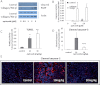
Although cyclooxygenase-2 (COX-2) inhibitors, such as the late stage development drug apricoxib, exhibit antitumor activity, their mechanisms of action have not been fully defined. In this study, we characterized the mechanisms of action of apricoxib in HT29 colorectal carcinoma. Apricoxib was weakly cytotoxic toward naive HT29 cells in vitro but inhibited tumor growth markedly in vivo. Pharmacokinetic analyses revealed that in vivo drug levels peaked at 2-4 µM and remained sufficient to completely inhibit prostaglandin E(2) production, but failed to reach concentrations cytotoxic for HT29 cells in monolayer culture. Despite this, apricoxib significantly inhibited tumor cell proliferation and induced apoptosis without affecting blood vessel density, although it did promote vascular normalization. Strikingly, apricoxib treatment induced a dose-dependent reversal of epithelial-mesenchymal transition (EMT), as shown by robust upregulation of E-cadherin and the virtual disappearance of vimentin and ZEB1 protein expression. In vitro, either anchorage-independent growth conditions or forced EMT sensitized HT29 and non-small cell lung cancer cells to apricoxib by 50-fold, suggesting that the occurrence of EMT may actually increase the dependence of colon and lung carcinoma cells on COX-2. Taken together, these data suggest that acquisition of mesenchymal characteristics sensitizes carcinoma cells to apricoxib resulting in significant single-agent antitumor activity.
Figures
Similar articles
-
Apricoxib, a novel inhibitor of COX-2, markedly improves standard therapy response in molecularly defined models of pancreatic cancer.Clin Cancer Res. 2012 Sep 15;18(18):5031-42. doi: 10.1158/1078-0432.CCR-12-0453. Epub 2012 Jul 24. Clin Cancer Res. 2012. PMID: 22829202 Free PMC article.
-
Apricoxib upregulates 15-PGDH and PGT in tobacco-related epithelial malignancies.Br J Cancer. 2012 Aug 7;107(4):707-12. doi: 10.1038/bjc.2012.203. Epub 2012 Jul 24. Br J Cancer. 2012. PMID: 22828609 Free PMC article.
-
Combination Treatment with Apricoxib and IL-27 Enhances Inhibition of Epithelial-Mesenchymal Transition in Human Lung Cancer Cells through a STAT1 Dominant Pathway.J Cancer Sci Ther. 2014 Nov;6(11):468-477. doi: 10.4172/1948-5956.1000310. Epub 2014 Nov 15. J Cancer Sci Ther. 2014. PMID: 26523208 Free PMC article.
-
Antitumor and anti-metastatic effects of cyclooxygenase-2 inhibition by celecoxib on human colorectal carcinoma xenografts in nude mouse rectum.Oncol Rep. 2012 Sep;28(3):777-84. doi: 10.3892/or.2012.1885. Epub 2012 Jun 26. Oncol Rep. 2012. PMID: 22751903 Free PMC article.
-
Efficient synthesis of apricoxib, CS-706, a selective cyclooxygenase-2 inhibitor, and evaluation of inhibition of prostaglandin E2 production in inflammatory breast cancer cells.Bioorg Med Chem Lett. 2011 Oct 15;21(20):6071-3. doi: 10.1016/j.bmcl.2011.08.050. Epub 2011 Aug 19. Bioorg Med Chem Lett. 2011. PMID: 21903394 Free PMC article.
Cited by
-
Metabolomics and EMT Markers of Breast Cancer: A Crosstalk and Future Perspective.Pathophysiology. 2022 May 27;29(2):200-222. doi: 10.3390/pathophysiology29020017. Pathophysiology. 2022. PMID: 35736645 Free PMC article. Review.
-
A randomized, placebo-controlled, multicenter, biomarker-selected, phase 2 study of apricoxib in combination with erlotinib in patients with advanced non-small-cell lung cancer.J Thorac Oncol. 2014 Apr;9(4):577-82. doi: 10.1097/JTO.0000000000000082. J Thorac Oncol. 2014. PMID: 24736085 Free PMC article. Clinical Trial.
-
Cyclooxygenase-2 Inhibition Potentiates the Efficacy of Vascular Endothelial Growth Factor Blockade and Promotes an Immune Stimulatory Microenvironment in Preclinical Models of Pancreatic Cancer.Mol Cancer Res. 2019 Feb;17(2):348-355. doi: 10.1158/1541-7786.MCR-18-0427. Epub 2018 Oct 17. Mol Cancer Res. 2019. PMID: 30333153 Free PMC article.
-
Apricoxib, a novel inhibitor of COX-2, markedly improves standard therapy response in molecularly defined models of pancreatic cancer.Clin Cancer Res. 2012 Sep 15;18(18):5031-42. doi: 10.1158/1078-0432.CCR-12-0453. Epub 2012 Jul 24. Clin Cancer Res. 2012. PMID: 22829202 Free PMC article.
-
Perioperative biobehavioral interventions to prevent cancer recurrence through combined inhibition of β-adrenergic and cyclooxygenase 2 signaling.Cancer. 2019 Jan 1;125(1):45-56. doi: 10.1002/cncr.31594. Epub 2018 Oct 6. Cancer. 2019. PMID: 30291805 Free PMC article. Review.
References
-
- Hanahan D, et al. (2011). Hallmarks of cancer: the next generation Cell 144 646–674 - PubMed
-
- De Groot D.J, et al. (2007). Non-steroidal anti-inflammatory drugs to potentiate chemotherapy effects: from lab to clinic Crit. Rev. Oncol. Hematol., 61 52–69 - PubMed
-
- Park S.W, et al. (2011). The effects of the stromal cell-derived cyclooxygenase-2 metabolite prostaglandin E2 on the proliferation of colon cancer cells J. Pharm. Exper. Therap ., 336 516–523 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

