Nanomaterial toxicity testing in the 21st century: use of a predictive toxicological approach and high-throughput screening
- PMID: 22676423
- PMCID: PMC4034475
- DOI: 10.1021/ar300022h
Nanomaterial toxicity testing in the 21st century: use of a predictive toxicological approach and high-throughput screening
Abstract
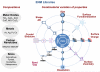
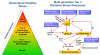
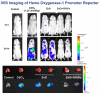
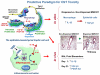
The production of engineered nanomaterials (ENMs) is a scientific breakthrough in material design and the development of new consumer products. While the successful implementation of nanotechnology is important for the growth of the global economy, we also need to consider the possible environmental health and safety (EHS) impact as a result of the novel physicochemical properties that could generate hazardous biological outcomes. In order to assess ENM hazard, reliable and reproducible screening approaches are needed to test the basic materials as well as nanoenabled products. A platform is required to investigate the potentially endless number of biophysicochemical interactions at the nano/bio interface, in response to which we have developed a predictive toxicological approach. We define a predictive toxicological approach as the use of mechanisms-based high-throughput screening in vitro to make predictions about the physicochemical properties of ENMs that may lead to the generation of pathology or disease outcomes in vivo. The in vivo results are used to validate and improve the in vitro high-throughput screening (HTS) and to establish structure-activity relationships (SARs) that allow hazard ranking and modeling by an appropriate combination of in vitro and in vivo testing. This notion is in agreement with the landmark 2007 report from the US National Academy of Sciences, "Toxicity Testing in the 21st Century: A Vision and a Strategy" (http://www.nap.edu/catalog.php?record_id=11970), which advocates increased efficiency of toxicity testing by transitioning from qualitative, descriptive animal testing to quantitative, mechanistic, and pathway-based toxicity testing in human cells or cell lines using high-throughput approaches. Accordingly, we have implemented HTS approaches to screen compositional and combinatorial ENM libraries to develop hazard ranking and structure-activity relationships that can be used for predicting in vivo injury outcomes. This predictive approach allows the bulk of the screening analysis and high-volume data generation to be carried out in vitro, following which limited, but critical, validation studies are carried out in animals or whole organisms. Risk reduction in the exposed human or environmental populations can then focus on limiting or avoiding exposures that trigger these toxicological responses as well as implementing safer design of potentially hazardous ENMs. In this Account, we review the tools required for establishing predictive toxicology paradigms to assess inhalation and environmental toxicological scenarios through the use of compositional and combinatorial ENM libraries, mechanism-based HTS assays, hazard ranking, and development of nano-SARs. We will discuss the major injury paradigms that have emerged based on specific ENM properties, as well as describing the safer design of ZnO nanoparticles based on characterization of dissolution chemistry as a major predictor of toxicity.
Figures








Similar articles
-
In silico analysis of nanomaterials hazard and risk.Acc Chem Res. 2013 Mar 19;46(3):802-12. doi: 10.1021/ar300049e. Epub 2012 Nov 8. Acc Chem Res. 2013. PMID: 23138971
-
Creative use of analytical techniques and high-throughput technology to facilitate safety assessment of engineered nanomaterials.Anal Bioanal Chem. 2018 Sep;410(24):6097-6111. doi: 10.1007/s00216-018-1289-y. Epub 2018 Aug 1. Anal Bioanal Chem. 2018. PMID: 30066194 Free PMC article. Review.
-
Nanomaterials in the environment: from materials to high-throughput screening to organisms.ACS Nano. 2011 Jan 25;5(1):13-20. doi: 10.1021/nn1034857. ACS Nano. 2011. PMID: 21261306 Review.
-
Implementation of alternative test strategies for the safety assessment of engineered nanomaterials.J Intern Med. 2013 Dec;274(6):561-77. doi: 10.1111/joim.12109. Epub 2013 Jul 24. J Intern Med. 2013. PMID: 23879741 Free PMC article. Review.
-
Ecological nanotoxicology: integrating nanomaterial hazard considerations across the subcellular, population, community, and ecosystems levels.Acc Chem Res. 2013 Mar 19;46(3):813-22. doi: 10.1021/ar300069t. Epub 2012 Oct 5. Acc Chem Res. 2013. PMID: 23039211
Cited by
-
Comprehensive insights into mechanism of nanotoxicity, assessment methods and regulatory challenges of nanomedicines.Discov Nano. 2024 Oct 4;19(1):165. doi: 10.1186/s11671-024-04118-1. Discov Nano. 2024. PMID: 39365367 Free PMC article. Review.
-
Wide pH, Adaptable High Internal Phase Pickering Emulsion Stabilized by a Crude Polysaccharide from Thesium chinense Turcz.Molecules. 2024 Sep 11;29(18):4312. doi: 10.3390/molecules29184312. Molecules. 2024. PMID: 39339307 Free PMC article.
-
In chemico methodology for engineered nanomaterial categorization according to number, nature and oxidative potential of reactive surface sites.Environ Sci Nano. 2024 Jul 9;11(9):3744-3760. doi: 10.1039/d3en00810j. eCollection 2024 Sep 12. Environ Sci Nano. 2024. PMID: 39280766 Free PMC article.
-
Differentially Induced Autophagy by Engineered Nanomaterial Treatment Has an Impact on Cellular Homeostasis and Cytotoxicity.Nano Lett. 2024 Sep 25;24(38):11793-11799. doi: 10.1021/acs.nanolett.4c01573. Epub 2024 Sep 13. Nano Lett. 2024. PMID: 39271139 Free PMC article.
-
Applicability of Quantum Dots in Breast Cancer Diagnostic and Therapeutic Modalities-A State-of-the-Art Review.Nanomaterials (Basel). 2024 Aug 31;14(17):1424. doi: 10.3390/nano14171424. Nanomaterials (Basel). 2024. PMID: 39269086 Free PMC article. Review.
References
-
- Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. - PubMed
-
- Nel AE, Madler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding biophysicochemical interactions at the nano-bio interface. Nature materials. 2009;8:543–57. - PubMed
-
- Xia T, Li N, Nel AE. Potential health impact of nanoparticles. Annual review of public health. 2009;30:137–50. - PubMed
-
- Meng H, Xia T, George S, Nel AE. A predictive toxicological paradigm for the safety assessment of nanomaterials. ACS nano. 2009;3:1620–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

