LSDP5 enhances triglyceride storage in hepatocytes by influencing lipolysis and fatty acid β-oxidation of lipid droplets
- PMID: 22675471
- PMCID: PMC3365886
- DOI: 10.1371/journal.pone.0036712
LSDP5 enhances triglyceride storage in hepatocytes by influencing lipolysis and fatty acid β-oxidation of lipid droplets
Abstract
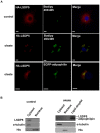
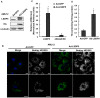
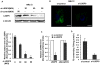
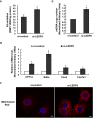
Lipid storage droplet protein 5 (LSDP5) is a lipid droplet-associated protein of the PAT (perilipin, adipophilin, and TIP47) family that is expressed in the liver in a peroxisome proliferator-activated receptor alpha (PPARα)-dependent manner; however, its exact function has not been elucidated. We noticed that LSDP5 was localized to the surface of lipid droplets in hepatocytes. Overexpression of LSDP5 enhanced lipid accumulation in the hepatic cell line AML12 and in primary hepatocytes. Knock-down of LSDP5 significantly decreased the triglyceride content of lipid droplets, stimulated lipolysis, and modestly increased the mitochondrial content and level of fatty-acid β-oxidation in the mitochondria. The expression of PPARα was increased in LSDP5-deficient cells and required for the increase in the level of fatty acid β-oxidation in LSDP5-deficient cells. Using serial deletions of LSDP5, we determined that the lipid droplet-targeting domain and the domain directing lipid droplet clustering overlapped and were localized to the 188 amino acid residues at the N-terminus of LSDP5. Our findings suggest that LSDP5, a novel lipid droplet protein, may contribute to triglyceride accumulation by negatively regulating lipolysis and fatty acid oxidation in hepatocytes.
Conflict of interest statement
Figures



Similar articles
-
LSDP5 is a PAT protein specifically expressed in fatty acid oxidizing tissues.Biochim Biophys Acta. 2007 Feb;1771(2):210-27. doi: 10.1016/j.bbalip.2006.11.011. Epub 2006 Dec 8. Biochim Biophys Acta. 2007. PMID: 17234449
-
PAT protein mRNA expression in primary rat hepatocytes: Effects of exposure to fatty acids.Int J Mol Med. 2010 Apr;25(4):505-12. doi: 10.3892/ijmm_00000370. Int J Mol Med. 2010. PMID: 20198297
-
Lipid storage droplet protein 5 reduces sodium palmitate‑induced lipotoxicity in human normal liver cells by regulating lipid metabolism‑related factors.Mol Med Rep. 2019 Aug;20(2):879-886. doi: 10.3892/mmr.2019.10360. Epub 2019 Jun 6. Mol Med Rep. 2019. PMID: 31173228 Free PMC article.
-
Lipid droplets in lipogenesis and lipolysis.Endocrinology. 2008 Mar;149(3):942-9. doi: 10.1210/en.2007-1713. Epub 2008 Jan 17. Endocrinology. 2008. PMID: 18202123 Review.
-
Perilipin 5, a lipid droplet protein adapted to mitochondrial energy utilization.Curr Opin Lipidol. 2014 Apr;25(2):110-7. doi: 10.1097/MOL.0000000000000057. Curr Opin Lipidol. 2014. PMID: 24535284 Free PMC article. Review.
Cited by
-
Perilipin 5 regulates islet lipid metabolism and insulin secretion in a cAMP-dependent manner: implication of its role in the postprandial insulin secretion.Diabetes. 2015 Apr;64(4):1299-310. doi: 10.2337/db14-0559. Epub 2014 Nov 12. Diabetes. 2015. PMID: 25392244 Free PMC article.
-
Cardiac-specific overexpression of perilipin 5 provokes severe cardiac steatosis via the formation of a lipolytic barrier.J Lipid Res. 2013 Apr;54(4):1092-102. doi: 10.1194/jlr.M034710. Epub 2013 Jan 23. J Lipid Res. 2013. PMID: 23345410 Free PMC article.
-
Understanding the Role of Perilipin 5 in Non-Alcoholic Fatty Liver Disease and Its Role in Hepatocellular Carcinoma: A Review of Novel Insights.Int J Mol Sci. 2021 May 17;22(10):5284. doi: 10.3390/ijms22105284. Int J Mol Sci. 2021. PMID: 34067931 Free PMC article. Review.
-
Nonalcoholic Fatty Liver Disease and Staging of Hepatic Fibrosis.Adv Exp Med Biol. 2024;1460:539-574. doi: 10.1007/978-3-031-63657-8_18. Adv Exp Med Biol. 2024. PMID: 39287864 Review.
-
Lipid droplets and steroidogenic cells.Exp Cell Res. 2016 Jan 15;340(2):209-14. doi: 10.1016/j.yexcr.2015.11.024. Epub 2015 Nov 27. Exp Cell Res. 2016. PMID: 26639173 Free PMC article. Review.
References
-
- Friedman JM. Obesity: Causes and control of excess body fat. Nature. 2009;459:340–342. - PubMed
-
- Unger RH. Lipotoxic diseases. Annu Rev Med. 2002;53:319–336. - PubMed
-
- Ducharme NA, Bickel PE. Lipid droplets in lipogenesis and lipolysis. Endocrinology. 2008;149:942–949. - PubMed
-
- Murphy DJ, Vance J. Mechanisms of lipid-body formation. Trends Biochem Sci. 1999;24:109–115. - PubMed
-
- Olofsson SO, Bostrom P, Andersson L, Rutberg M, Perman J, et al. Lipid droplets as dynamic organelles connecting storage and efflux of lipids. Biochim Biophys Acta. 2009;1791:448–458. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

