Immunofluorescence-guided atomic force microscopy to measure the micromechanical properties of the pericellular matrix of porcine articular cartilage
- PMID: 22675162
- PMCID: PMC3479909
- DOI: 10.1098/rsif.2012.0314
Immunofluorescence-guided atomic force microscopy to measure the micromechanical properties of the pericellular matrix of porcine articular cartilage
Abstract
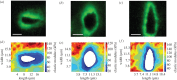
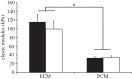
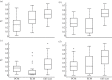
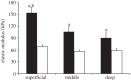
The pericellular matrix (PCM) is a narrow region that is rich in type VI collagen that surrounds each chondrocyte within the extracellular matrix (ECM) of articular cartilage. Previous studies have demonstrated that the chondrocyte micromechanical environment depends on the relative properties of the chondrocyte, its PCM and the ECM. The objective of this study was to measure the influence of type VI collagen on site-specific micromechanical properties of cartilage in situ by combining atomic force microscopy stiffness mapping with immunofluorescence imaging of PCM and ECM regions in cryo-sectioned tissue samples. This method was used to test the hypotheses that PCM biomechanical properties correlate with the presence of type VI collagen and are uniform with depth from the articular surface. Control experiments verified that immunolabelling did not affect the properties of the ECM or PCM. PCM biomechanical properties correlated with the presence of type VI collagen, and matrix regions lacking type VI collagen immediately adjacent to the PCM exhibited higher elastic moduli than regions positive for type VI collagen. PCM elastic moduli were similar in all three zones. Our findings provide further support for type VI collagen in defining the chondrocyte PCM and contributing to its biological and biomechanical properties.
Figures



Similar articles
-
A biomechanical role for perlecan in the pericellular matrix of articular cartilage.Matrix Biol. 2012 Jul;31(6):320-7. doi: 10.1016/j.matbio.2012.05.002. Epub 2012 May 30. Matrix Biol. 2012. PMID: 22659389 Free PMC article.
-
Depth-dependent anisotropy of the micromechanical properties of the extracellular and pericellular matrices of articular cartilage evaluated via atomic force microscopy.J Biomech. 2013 Feb 1;46(3):586-92. doi: 10.1016/j.jbiomech.2012.09.003. Epub 2012 Oct 11. J Biomech. 2013. PMID: 23062866 Free PMC article.
-
Micromechanical mapping of early osteoarthritic changes in the pericellular matrix of human articular cartilage.Osteoarthritis Cartilage. 2013 Dec;21(12):1895-903. doi: 10.1016/j.joca.2013.08.026. Epub 2013 Sep 8. Osteoarthritis Cartilage. 2013. PMID: 24025318 Free PMC article.
-
The pericellular matrix as a transducer of biomechanical and biochemical signals in articular cartilage.Ann N Y Acad Sci. 2006 Apr;1068:498-512. doi: 10.1196/annals.1346.011. Ann N Y Acad Sci. 2006. PMID: 16831947 Review.
-
The structure and function of the pericellular matrix of articular cartilage.Matrix Biol. 2014 Oct;39:25-32. doi: 10.1016/j.matbio.2014.08.009. Epub 2014 Aug 27. Matrix Biol. 2014. PMID: 25172825 Free PMC article. Review.
Cited by
-
Perlecan in Pericellular Mechanosensory Cell-Matrix Communication, Extracellular Matrix Stabilisation and Mechanoregulation of Load-Bearing Connective Tissues.Int J Mol Sci. 2021 Mar 8;22(5):2716. doi: 10.3390/ijms22052716. Int J Mol Sci. 2021. PMID: 33800241 Free PMC article. Review.
-
High resistance of the mechanical properties of the chondrocyte pericellular matrix to proteoglycan digestion by chondroitinase, aggrecanase, or hyaluronidase.J Mech Behav Biomed Mater. 2014 Oct;38:183-97. doi: 10.1016/j.jmbbm.2013.09.021. Epub 2013 Oct 3. J Mech Behav Biomed Mater. 2014. PMID: 24156881 Free PMC article.
-
AFM-Based Method for Measurement of Normal and Osteoarthritic Human Articular Cartilage Surface Roughness.Materials (Basel). 2020 May 16;13(10):2302. doi: 10.3390/ma13102302. Materials (Basel). 2020. PMID: 32429426 Free PMC article.
-
Biomimetic Proteoglycans Strengthen the Pericellular Matrix of Normal and Osteoarthritic Human Cartilage.ACS Biomater Sci Eng. 2024 Sep 9;10(9):5617-5623. doi: 10.1021/acsbiomaterials.4c00813. Epub 2024 Aug 12. ACS Biomater Sci Eng. 2024. PMID: 39133208 Free PMC article.
-
Osteoarthritis as a disease of the cartilage pericellular matrix.Matrix Biol. 2018 Oct;71-72:40-50. doi: 10.1016/j.matbio.2018.05.008. Epub 2018 May 22. Matrix Biol. 2018. PMID: 29800616 Free PMC article. Review.
References
-
- Mow V. C., Bachrach N. M., Setton L. A., Guilak F. 1994. Stress, strain, pressure, and flow fields in articular cartilage and chondrocytes. In Cell mechanics and cellular engineering (eds Mow V. C., Guilak F., Tran-Son-Tay R., Hochmuth R. M.), pp. 345–379 New York, NY: Springer
-
- Lai W. M., Sun D. D., Ateshian G. A., Guo X. E., Mow V. C. 2002. Electrical signals for chondrocytes in cartilage. Biorheology 39, 39–45 - PubMed
-
- Mow V. C., Guo X. E. 2002. Mechano-electrochemical properties of articular cartilage: their inhomogeneities and anisotropies. Annu. Rev. Biomed. Eng. 4, 175–20910.1146/annurev.bioeng.4.110701.120309 (doi:10.1146/annurev.bioeng.4.110701.120309) - DOI - DOI - PubMed
-
- Guilak F., Sah R. L., Setton L. A. 1997. Physical regulation of cartilage metabolism. In Basic orthopaedic biomechanics, 2nd edn. (eds Mow V. C., Hayes W. C.), pp. 179–207 Philadelphia, PA: Lippincott-Raven Publishers
-
- Williams G. M., Chan E. F., Temple-Wong M. M., Bae W. C., Masuda K., Bugbee W. D., Sah R. L. 2010. Shape, loading, and motion in the bioengineering design, fabrication, and testing of personalized synovial joints. J. Biomech. 43, 156–16510.1016/j.jbiomech.2009.09.021 (doi:10.1016/j.jbiomech.2009.09.021) - DOI - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

