Cytosine-phosphate-guanosine-DNA induces CD274 expression in human B cells and suppresses T helper type 2 cytokine production in pollen antigen-stimulated CD4-positive cells
- PMID: 22670772
- PMCID: PMC3390467
- DOI: 10.1111/j.1365-2249.2012.04585.x
Cytosine-phosphate-guanosine-DNA induces CD274 expression in human B cells and suppresses T helper type 2 cytokine production in pollen antigen-stimulated CD4-positive cells
Abstract
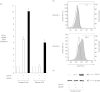
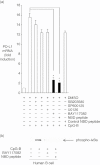
Co-stimulatory molecules are important for regulating T cell activation and immune response. CD274 [programmed death ligand 1 (PD-L1), B7-H1] has emerged as an important immune modulator that can block T cell receptor signalling. We have investigated whether PD-L1 and other co-stimulatory ligands could be expressed in human B cells stimulated by cytosine-phosphate-guanosine (CpG)-DNA. CpG-DNA strongly induced the co-inhibitory molecule ligand, PD-L1, of human B cells. Results show that nuclear factor-kappa B (NF-κB) signalling is involved directly in CpG-DNA-induced PD-L1 expression in human B cells. We sought to determine the effect of CpG-DNA-treated B cells on T helper type 2 (Th2) cytokine production in Cry j 1 (Japanese pollen antigen)-stimulated human CD4-positive cells from patients with seasonal allergic rhinitis caused by Japanese cedar pollen. CpG-DNA-treated B cells reduced Cry j 1-induced interleukin (IL)-5 and IL-13 production in CD4-positive cells. When the binding of PD-1 to PD-L1 was inhibited by PD-1-immunoglobulin (Ig), this chimera molecule reversed the previously described reductions in IL-5 and IL-13 production. In contrast, the CpG B-treated B cells increased both interferon (IFN)-γ and IL-12 production in the presence of Cry j 1-stimulated CD4-positive cells. CpG-DNA simultaneously reduced the expression of B7RP-1 [also known as inducible co-stimulator ligand (ICOSL), B7-H2] and the ligand of CD30 (CD30L). These results indicate that CpG-DNA induces co-inhibitory molecule ligand PD-L1 expression in human B cells and PD-L1 can suppress Th2 cytokine production in Cry j 1-stimulated CD4-positive cells, while CpG-DNA increased Th1 cytokine production and reduced the expression of co-stimulatory molecule ligands that can promote Th2 inflammatory responses.
© 2012 The Authors. Clinical and Experimental Immunology © 2012 British Society for Immunology.
Figures

Similar articles
-
Defective suppression of Th2 cytokines by CD4CD25 regulatory T cells in birch allergics during birch pollen season.Clin Exp Allergy. 2004 Sep;34(9):1364-72. doi: 10.1111/j.1365-2222.2004.02067.x. Clin Exp Allergy. 2004. PMID: 15347368
-
Th1/Th2 response profiles to the major allergens Cry j 1 and Cry j 2 of Japanese cedar pollen.Allergy. 1996 Oct;51(10):732-40. doi: 10.1111/j.1398-9995.1996.tb02118.x. Allergy. 1996. PMID: 8905002
-
Roles of carbohydrates on Cry j 1, the major allergen of Japanese cedar pollen, in specific T-cell responses.J Allergy Clin Immunol. 2001 Jul;108(1):101-8. doi: 10.1067/mai.2001.115757. J Allergy Clin Immunol. 2001. PMID: 11447389
-
Peptide immunotherapy for allergic diseases using a rice-based edible vaccine.Curr Opin Allergy Clin Immunol. 2006 Dec;6(6):455-60. doi: 10.1097/01.all.0000246621.34247.fa. Curr Opin Allergy Clin Immunol. 2006. PMID: 17088651 Review.
-
New expression of PD-L1 on activated CD4+ T cells opens up new opportunities for cell interactions and signaling.Hum Immunol. 2024 Jul;85(4):110831. doi: 10.1016/j.humimm.2024.110831. Epub 2024 Jun 12. Hum Immunol. 2024. PMID: 38870593 Review.
Cited by
-
Soluble CD80 Protein Delays Tumor Growth and Promotes Tumor-Infiltrating Lymphocytes.Cancer Immunol Res. 2018 Jan;6(1):59-68. doi: 10.1158/2326-6066.CIR-17-0026. Epub 2017 Nov 9. Cancer Immunol Res. 2018. PMID: 29122838 Free PMC article.
-
Identification of key genes in allergic rhinitis by bioinformatics analysis.J Int Med Res. 2021 Jul;49(7):3000605211029521. doi: 10.1177/03000605211029521. J Int Med Res. 2021. PMID: 34334005 Free PMC article.
-
PD-L1+ Regulatory B Cells Are Significantly Decreased in Rheumatoid Arthritis Patients and Increase After Successful Treatment.Front Immunol. 2018 Oct 1;9:2241. doi: 10.3389/fimmu.2018.02241. eCollection 2018. Front Immunol. 2018. PMID: 30327652 Free PMC article.
-
Allergen Immunotherapy: Current and Future Trends.Cells. 2022 Jan 8;11(2):212. doi: 10.3390/cells11020212. Cells. 2022. PMID: 35053328 Free PMC article. Review.
-
Immunosuppressive Mechanisms of Regulatory B Cells.Front Immunol. 2021 Apr 29;12:611795. doi: 10.3389/fimmu.2021.611795. eCollection 2021. Front Immunol. 2021. PMID: 33995344 Free PMC article. Review.
References
-
- Folkl A, Bienzle D. Structure and function of programmed death (PD) molecules. Vet Immunol Immunopathol. 2010;134:33–8. - PubMed
-
- Allam J, Peng W, Appel T, et al. Toll-like receptor 4 ligation enforces tolerogenic properties of oral mucosal Langerhans cells. J Allergy Clin Immunol. 2008;121:368–74. - PubMed
-
- Liu N, Ohnishi N, Ni L, Akira S, Bacon K. CpG directly induces T-bet expression and inhibits IgG1 and IgE switching in B cells. Nat Immunol. 2003;4:687–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

