Mdm2 RING mutation enhances p53 transcriptional activity and p53-p300 interaction
- PMID: 22666487
- PMCID: PMC3362553
- DOI: 10.1371/journal.pone.0038212
Mdm2 RING mutation enhances p53 transcriptional activity and p53-p300 interaction
Abstract
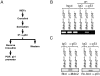
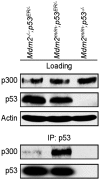
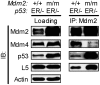
The p53 transcription factor and tumor suppressor is regulated primarily by the E3 ubiquitin ligase Mdm2, which ubiquitinates p53 to target it for proteasomal degradation. Aside from its ubiquitin ligase function, Mdm2 has been believed to be capable of suppressing p53's transcriptional activity by binding with and masking the transactivation domain of p53. The ability of Mdm2 to restrain p53 activity by binding alone, without ubiquitination, was challenged by a 2007 study using a knockin mouse harboring a single cysteine-to-alanine point mutation (C462A) in Mdm2's RING domain. Mouse embryonic fibroblasts with this mutation, which abrogates Mdm2's E3 ubiquitin ligase activity without affecting its ability to bind with p53, were unable to suppress p53 activity. In this study, we utilized the Mdm2(C462A) mouse model to characterize in further detail the role of Mdm2's RING domain in the control of p53. Here, we show in vivo that the Mdm2(C462A) protein not only fails to suppress p53, but compared to the complete absence of Mdm2, Mdm2(C462A) actually enhances p53 transcriptional activity toward p53 target genes p21/CDKN1A, MDM2, BAX, NOXA, and 14-3-3σ. In addition, we found that Mdm2(C462A) facilitates the interaction between p53 and the acetyltransferase CBP/p300, and it fails to heterodimerize with its homolog and sister regulator of p53, Mdmx, suggesting that a fully intact RING domain is required for Mdm2's inhibition of the p300-p53 interaction and for its interaction with Mdmx. These findings help us to better understand the complex regulation of the Mdm2-p53 pathway and have important implications for chemotherapeutic agents targeting Mdm2, as they suggest that inhibition of Mdm2's E3 ubiquitin ligase activity may be sufficient for increasing p53 activity in vivo, without the need to block Mdm2-p53 binding.
Conflict of interest statement
Figures

Similar articles
-
Inactivation of the MDM2 RING domain enhances p53 transcriptional activity in mice.J Biol Chem. 2017 Dec 29;292(52):21614-21622. doi: 10.1074/jbc.RA117.000122. Epub 2017 Nov 9. J Biol Chem. 2017. PMID: 29123033 Free PMC article.
-
Unlocking the Mdm2-p53 loop: ubiquitin is the key.Cell Cycle. 2008 Feb 1;7(3):287-92. doi: 10.4161/cc.7.3.5358. Epub 2007 Nov 25. Cell Cycle. 2008. PMID: 18235222 Review.
-
Targeted inactivation of Mdm2 RING finger E3 ubiquitin ligase activity in the mouse reveals mechanistic insights into p53 regulation.Cancer Cell. 2007 Oct;12(4):355-66. doi: 10.1016/j.ccr.2007.09.007. Cancer Cell. 2007. PMID: 17936560
-
Structural analysis of MDM2 RING separates degradation from regulation of p53 transcription activity.Nat Struct Mol Biol. 2017 Jul;24(7):578-587. doi: 10.1038/nsmb.3414. Epub 2017 May 29. Nat Struct Mol Biol. 2017. PMID: 28553961 Free PMC article.
-
Escape, or Vanish: Control the Fate of p53 through MDM2-Mediated Ubiquitination.Anticancer Agents Med Chem. 2015;16(2):174-89. doi: 10.2174/1871520615666150907093358. Anticancer Agents Med Chem. 2015. PMID: 26343143 Review.
Cited by
-
Rhythmic expression of DEC2 protein in vitro and in vivo.Biomed Rep. 2016 Jun;4(6):704-710. doi: 10.3892/br.2016.656. Epub 2016 Apr 14. Biomed Rep. 2016. PMID: 27284410 Free PMC article.
-
The uric acid transporter SLC2A9 is a direct target gene of the tumor suppressor p53 contributing to antioxidant defense.Oncogene. 2015 Apr 2;34(14):1799-810. doi: 10.1038/onc.2014.119. Epub 2014 May 26. Oncogene. 2015. PMID: 24858040
-
Regulation of p53 by Mdm2 E3 ligase function is dispensable in embryogenesis and development, but essential in response to DNA damage.Cancer Cell. 2014 Aug 11;26(2):235-47. doi: 10.1016/j.ccr.2014.06.006. Cancer Cell. 2014. PMID: 25117711 Free PMC article.
-
Inactivation of the MDM2 RING domain enhances p53 transcriptional activity in mice.J Biol Chem. 2017 Dec 29;292(52):21614-21622. doi: 10.1074/jbc.RA117.000122. Epub 2017 Nov 9. J Biol Chem. 2017. PMID: 29123033 Free PMC article.
-
Targeting p53-MDM2-MDMX loop for cancer therapy.Subcell Biochem. 2014;85:281-319. doi: 10.1007/978-94-017-9211-0_16. Subcell Biochem. 2014. PMID: 25201201 Free PMC article. Review.
References
-
- Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer. 2006;6:909–923. - PubMed
-
- Yang Y, Li CC, Weissman AM. Regulating the p53 system through ubiquitination. Oncogene. 2004;23:2096–2106. - PubMed
-
- Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. - PubMed
-
- Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Letter. 1997;420:25–27. - PubMed
-
- Kubbutat MHG, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

