Aldosterone inhibits the fetal program and increases hypertrophy in the heart of hypertensive mice
- PMID: 22666483
- PMCID: PMC3364229
- DOI: 10.1371/journal.pone.0038197
Aldosterone inhibits the fetal program and increases hypertrophy in the heart of hypertensive mice
Abstract
Background: Arterial hypertension (AH) induces cardiac hypertrophy and reactivation of "fetal" gene expression. In rodent heart, alpha-Myosin Heavy Chain (MyHC) and its micro-RNA miR-208a regulate the expression of beta-MyHC and of its intronic miR-208b. However, the role of aldosterone in these processes remains unclear.
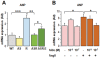
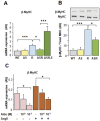
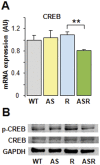
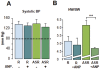
Methodology/principal findings: RT-PCR and western-blot were used to investigate the genes modulated by arterial hypertension and cardiac hyperaldosteronism. We developed a model of double-transgenic mice (AS-Ren) with cardiac hyperaldosteronism (AS mice) and systemic hypertension (Ren). AS-Ren mice had increased (x2) angiotensin II in plasma and increased (x2) aldosterone in heart. Ren and AS-Ren mice had a robust and similar hypertension (+70%) versus their controls. Anatomical data and echocardiography showed a worsening of cardiac hypertrophy (+41%) in AS-Ren mice (P<0.05 vs Ren). The increase of ANP (x 2.5; P<0.01) mRNA observed in Ren mice was blunted in AS-Ren mice. This non-induction of antitrophic natriuretic peptides may be involved in the higher trophic cardiac response in AS-Ren mice, as indicated by the markedly reduced cardiac hypertrophy in ANP-infused AS-Ren mice for one month. Besides, the AH-induced increase of ßMyHC and its intronic miRNA-208b was prevented in AS-Ren. The inhibition of miR 208a (-75%, p<0.001) in AS-Ren mice compared to AS was associated with increased Sox 6 mRNA (x 1.34; p<0.05), an inhibitor of ßMyHC transcription. Eplerenone prevented all aldosterone-dependent effects.
Conclusions/significance: Our results indicate that increased aldosterone in heart inhibits the induction of atrial natriuretic peptide expression, via the mineralocorticoid receptor. This worsens cardiac hypertrophy without changing blood pressure. Moreover, this work reveals an original aldosterone-dependent inhibition of miR-208a in hypertension, resulting in the inhibition of β-myosin heavy chain expression through the induction of its transcriptional repressor Sox6. Thus, aldosterone inhibits the fetal program and increases cardiac hypertrophy in hypertensive mice.
Conflict of interest statement
Figures
Similar articles
-
Aldosterone inhibits antifibrotic factors in mouse hypertensive heart.Hypertension. 2012 Jun;59(6):1179-87. doi: 10.1161/HYPERTENSIONAHA.111.190512. Epub 2012 Apr 30. Hypertension. 2012. PMID: 22547442
-
MiRNA-208a and miRNA-208b are triggered in thyroid hormone-induced cardiac hypertrophy - role of type 1 Angiotensin II receptor (AT1R) on miRNA-208a/α-MHC modulation.Mol Cell Endocrinol. 2013 Jul 15;374(1-2):117-24. doi: 10.1016/j.mce.2013.04.010. Epub 2013 Apr 26. Mol Cell Endocrinol. 2013. PMID: 23623871
-
MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice.J Clin Invest. 2009 Sep;119(9):2772-86. doi: 10.1172/JCI36154. Epub 2009 Aug 10. J Clin Invest. 2009. PMID: 19726871 Free PMC article.
-
Myocyte-specific enhancer factor 2C: a novel target gene of miR-214-3p in suppressing angiotensin II-induced cardiomyocyte hypertrophy.Sci Rep. 2016 Oct 31;6:36146. doi: 10.1038/srep36146. Sci Rep. 2016. PMID: 27796324 Free PMC article.
-
The emerging role of miR-208a in the heart.DNA Cell Biol. 2013 Jan;32(1):8-12. doi: 10.1089/dna.2012.1787. Epub 2012 Nov 2. DNA Cell Biol. 2013. PMID: 23121236 Review.
Cited by
-
Aldosterone and cardiovascular disease: the heart of the matter.Trends Endocrinol Metab. 2013 Jan;24(1):21-30. doi: 10.1016/j.tem.2012.09.004. Epub 2012 Oct 3. Trends Endocrinol Metab. 2013. PMID: 23040074 Free PMC article. Review.
-
Common variants in TGFBR2 and miR-518 genes are associated with hypertension in the Chinese population.Am J Hypertens. 2014 Oct;27(10):1268-76. doi: 10.1093/ajh/hpu047. Epub 2014 Mar 31. Am J Hypertens. 2014. PMID: 24687999 Free PMC article.
-
Sox6, A Potential Target for MicroRNAs in Cardiometabolic Disease.Curr Hypertens Rep. 2022 May;24(5):145-156. doi: 10.1007/s11906-022-01175-8. Epub 2022 Feb 5. Curr Hypertens Rep. 2022. PMID: 35124768 Review.
-
Prediction of single-cell mechanisms for disease progression in hypertrophic remodelling by a trans-omics approach.Sci Rep. 2021 Apr 14;11(1):8112. doi: 10.1038/s41598-021-86821-y. Sci Rep. 2021. PMID: 33854108 Free PMC article.
-
Myosins and MyomiR Network in Patients with Obstructive Hypertrophic Cardiomyopathy.Biomedicines. 2022 Sep 3;10(9):2180. doi: 10.3390/biomedicines10092180. Biomedicines. 2022. PMID: 36140281 Free PMC article.
References
-
- Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999;79:215–262. - PubMed
-
- Brilla CG, Weber KT. Mineralocorticoid excess, dietary sodium, and myocardial fibrosis. J Lab Clin Med. 1992;120:893–901. - PubMed
-
- Robert V, Van Thiem N, Cheav SL, Mouas C, Swynghedauw B, et al. Increased cardiac types I and III collagen mRNAs in aldosterone-salt hypertension. Hypertension. 1994;24:30–36. - PubMed
-
- Zannad F, Alla F, Dousset B, Perez A, Pitt B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES). Rales Investigators. Circulation. 2000;102:2700–2706. - PubMed
-
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

