A general strategy to endow natural fusion-protein-derived peptides with potent antiviral activity
- PMID: 22666328
- PMCID: PMC3353973
- DOI: 10.1371/journal.pone.0036833
A general strategy to endow natural fusion-protein-derived peptides with potent antiviral activity
Abstract
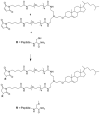
Fusion between the viral and target cell membranes is an obligatory step for the infectivity of all enveloped virus, and blocking this process is a clinically validated therapeutic strategy.Viral fusion is driven by specialized proteins which, although specific to each virus, act through a common mechanism, the formation of a complex between two heptad repeat (HR) regions. The HR regions are initially separated in an intermediate termed "prehairpin", which bridges the viral and cell membranes, and then fold onto each other to form a 6-helical bundle (6HB), driving the two membranes to fuse. HR-derived peptides can inhibit viral infectivity by binding to the prehairpin intermediate and preventing its transition to the 6HB.The antiviral activity of HR-derived peptides differs considerably among enveloped viruses. For weak inhibitors, potency can be increased by peptide engineering strategies, but sequence-specific optimization is time-consuming. In seeking ways to increase potency without changing the native sequence, we previously reported that attachment to the HR peptide of a cholesterol group ("cholesterol-tagging") dramatically increases its antiviral potency, and simultaneously increases its half-life in vivo. We show here that antiviral potency may be increased by combining cholesterol-tagging with dimerization of the HR-derived sequence, using as examples human parainfluenza virus, Nipah virus, and HIV-1. Together, cholesterol-tagging and dimerization may represent strategies to boost HR peptide potency to levels that in some cases may be compatible with in vivo use, possibly contributing to emergency responses to outbreaks of existing or novel viruses.
Conflict of interest statement
Figures
Similar articles
-
Broad spectrum antiviral activity for paramyxoviruses is modulated by biophysical properties of fusion inhibitory peptides.Sci Rep. 2017 Mar 8;7:43610. doi: 10.1038/srep43610. Sci Rep. 2017. PMID: 28344321 Free PMC article.
-
Cholesterol-conjugated peptide antivirals: a path to a rapid response to emerging viral diseases.J Pept Sci. 2015 May;21(5):379-86. doi: 10.1002/psc.2706. Epub 2014 Oct 20. J Pept Sci. 2015. PMID: 25331523 Free PMC article. Review.
-
Molecular determinants of antiviral potency of paramyxovirus entry inhibitors.J Virol. 2007 Oct;81(19):10567-74. doi: 10.1128/JVI.01181-07. Epub 2007 Jul 25. J Virol. 2007. PMID: 17652384 Free PMC article.
-
Engineering Protease-Resistant Peptides to Inhibit Human Parainfluenza Viral Respiratory Infection.J Am Chem Soc. 2021 Apr 21;143(15):5958-5966. doi: 10.1021/jacs.1c01565. Epub 2021 Apr 7. J Am Chem Soc. 2021. PMID: 33825470 Free PMC article.
-
Prospects and strategies for the discovery and development of small-molecule inhibitors of six-helix bundle formation in class 1 viral fusion proteins.Curr Opin Investig Drugs. 2006 Feb;7(2):118-27. Curr Opin Investig Drugs. 2006. PMID: 16499281 Review.
Cited by
-
Unity in diversity: shared mechanism of entry among paramyxoviruses.Prog Mol Biol Transl Sci. 2015;129:1-32. doi: 10.1016/bs.pmbts.2014.10.001. Epub 2014 Dec 1. Prog Mol Biol Transl Sci. 2015. PMID: 25595799 Free PMC article. Review.
-
Combining 25-Hydroxycholesterol with an HIV Fusion Inhibitor Peptide: Interaction with Biomembrane Model Systems and Human Blood Cells.ACS Infect Dis. 2019 Apr 12;5(4):582-591. doi: 10.1021/acsinfecdis.8b00321. Epub 2019 Feb 28. ACS Infect Dis. 2019. PMID: 30816690 Free PMC article.
-
Fatal measles virus infection prevented by brain-penetrant fusion inhibitors.J Virol. 2013 Dec;87(24):13785-94. doi: 10.1128/JVI.02436-13. Epub 2013 Oct 9. J Virol. 2013. PMID: 24109233 Free PMC article.
-
Broad spectrum antiviral activity for paramyxoviruses is modulated by biophysical properties of fusion inhibitory peptides.Sci Rep. 2017 Mar 8;7:43610. doi: 10.1038/srep43610. Sci Rep. 2017. PMID: 28344321 Free PMC article.
-
Dual Inhibition of Human Parainfluenza Type 3 and Respiratory Syncytial Virus Infectivity with a Single Agent.J Am Chem Soc. 2019 Aug 14;141(32):12648-12656. doi: 10.1021/jacs.9b04615. Epub 2019 Aug 5. J Am Chem Soc. 2019. PMID: 31268705 Free PMC article.
References
-
- Eckert DM, Kim PS. Mechanisms of viral membrane fusion and its inhibition. Annu Rev Biochem. 2001;70:777–810. - PubMed
-
- LaBonte J, Lebbos J, Kirkpatrick P. Enfuvirtide. Nat Rev Drug Discov. 2003;2:345–346. - PubMed
-
- Young JK, Hicks RP, Wright GE, Morrison TG. Analysis of a peptide inhibitor of paramyxovirus (NDV) fusion using biological assays, NMR, and molecular modeling. Virology. 1997;238:291–304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U19 AI76982/AI/NIAID NIH HHS/United States
- R01 AI076335/AI/NIAID NIH HHS/United States
- U54 AI057158/AI/NIAID NIH HHS/United States
- #R21NS076385/NS/NINDS NIH HHS/United States
- #R01AI31971/AI/NIAID NIH HHS/United States
- R01 AI031971/AI/NIAID NIH HHS/United States
- #R01AI076335/AI/NIAID NIH HHS/United States
- R21 AI090354/AI/NIAID NIH HHS/United States
- #U54AI057158/AI/NIAID NIH HHS/United States
- #R21AI090354/AI/NIAID NIH HHS/United States
- U19 AI076982/AI/NIAID NIH HHS/United States
- R21 NS076385/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources

