Antitumor activity of celastrol nanoparticles in a xenograft retinoblastoma tumor model
- PMID: 22661892
- PMCID: PMC3357982
- DOI: 10.2147/IJN.S29945
Antitumor activity of celastrol nanoparticles in a xenograft retinoblastoma tumor model
Abstract
Background: Celastrol, a Chinese herbal medicine, has shown antitumor activity against various tumor cell lines. However, the effect of celastrol on retinoblastoma has not yet been analyzed. Additionally, the poor water solubility of celastrol restricts further therapeutic applications. The goal of this study was to evaluate the effect of celastrol nanoparticles (CNPs) on retinoblastoma and to investigate the potential mechanisms involved.
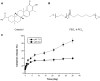
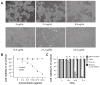
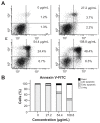
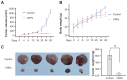
Methods: Celastrol-loaded poly(ethylene glycol)-block-poly(ɛ-caprolactone) nanopolymeric micelles were developed to improve the hydrophilicity of celastrol. The 2-(2-methoxy-4- nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulf-ophenyl)-2H tetrazolium monosodium salt (WST-8) assay was used to determine the inhibitory effect of CNPs on SO-Rb 50 cell proliferation in vitro. Immunofluorescence was used to evaluate the apoptotic effect of CNPs on nuclear morphology, and flow cytometry was used to quantify cellular apoptosis. The expression of Bcl-2, Bax, NF-κB p65, and phospo-NF-κB p65 proteins was assessed by Western blotting. A human retinoblastoma xenograft model was used to evaluate the inhibitory effects of CNPs on retinoblastoma in NOD-SCID mice. Hematoxylin and eosin staining was used to assess the apoptotic effects of CNPs on retinoblastoma.
Results: CNPs inhibit the proliferation of SO-Rb 50 cells in a dose- and time-dependent manner with an IC(50) of 17.733 μg/mL (celastrol-loading content: 7.36%) after exposure to CNPs for 48 hours. CNPs induce apoptosis in SO-Rb 50 cells in a dose-dependent manner. The expression of Bcl-2, NF-κB p65, and phospo-NF-κB p65 proteins decreased after exposure to CNPs 54.4 μg/mL for 48 hours. Additionally, the Bax/Bcl-2 ratio increased, whereas the expression of Bax itself was not significantly altered. CNPs inhibit the growth of retinoblastoma and induce apoptosis in retinoblastoma cells in mice.
Conclusion: CNPs inhibit the growth of retinoblastoma in mouse xenograft model by inducing apoptosis in SO-Rb 50 cells, which may be related to the increased Bax/Bcl-2 ratio and the inhibition of NF-κB. CNPs may represent a potential alternative treatment for retinoblastoma.
Keywords: SO-Rb 50 cells; apoptosis; celastrol nanoparticles; nanopolymeric micelles; poly(ethylene glycol)-block-poly(ɛ-caprolactone).
Figures



Similar articles
-
Effectively suppressed angiogenesis-mediated retinoblastoma growth using celastrol nanomicelles.Drug Deliv. 2020 Dec;27(1):358-366. doi: 10.1080/10717544.2020.1730522. Drug Deliv. 2020. PMID: 32091275 Free PMC article.
-
Celastrol nanoparticles inhibit corneal neovascularization induced by suturing in rats.Int J Nanomedicine. 2012;7:1163-73. doi: 10.2147/IJN.S27860. Epub 2012 Mar 1. Int J Nanomedicine. 2012. PMID: 22419865 Free PMC article.
-
NF-kappa B modulation is involved in celastrol induced human multiple myeloma cell apoptosis.PLoS One. 2014 Apr 22;9(4):e95846. doi: 10.1371/journal.pone.0095846. eCollection 2014. PLoS One. 2014. PMID: 24755677 Free PMC article.
-
Celastrol as an emerging anticancer agent: Current status, challenges and therapeutic strategies.Biomed Pharmacother. 2023 Jul;163:114882. doi: 10.1016/j.biopha.2023.114882. Epub 2023 May 15. Biomed Pharmacother. 2023. PMID: 37196541 Review.
-
Thiazolidinedione-Based Structure Modification of Celastrol Provides Thiazolidinedione-Conjugated Derivatives as Potent Agents against Non-Small-Cell Lung Cancer Cells through a Mitochondria-Mediated Apoptotic Pathway.J Nat Prod. 2022 Apr 22;85(4):1147-1156. doi: 10.1021/acs.jnatprod.2c00104. Epub 2022 Mar 7. J Nat Prod. 2022. PMID: 35255689 Review.
Cited by
-
Nanotechnology for Pediatric Retinoblastoma Therapy.Pharmaceuticals (Basel). 2022 Aug 31;15(9):1087. doi: 10.3390/ph15091087. Pharmaceuticals (Basel). 2022. PMID: 36145308 Free PMC article. Review.
-
Optimization on biodistribution and antitumor activity of tripterine using polymeric nanoparticles through RES saturation.Drug Deliv. 2017 Nov;24(1):1891-1897. doi: 10.1080/10717544.2017.1410260. Drug Deliv. 2017. PMID: 29191042 Free PMC article.
-
Tripterygium hypoglaucum (Lévl.) Hutch and Its Main Bioactive Components: Recent Advances in Pharmacological Activity, Pharmacokinetics and Potential Toxicity.Front Pharmacol. 2021 Nov 23;12:715359. doi: 10.3389/fphar.2021.715359. eCollection 2021. Front Pharmacol. 2021. PMID: 34887747 Free PMC article. Review.
-
Effectively suppressed angiogenesis-mediated retinoblastoma growth using celastrol nanomicelles.Drug Deliv. 2020 Dec;27(1):358-366. doi: 10.1080/10717544.2020.1730522. Drug Deliv. 2020. PMID: 32091275 Free PMC article.
-
Nanotechnology: an effective tool for enhancing bioavailability and bioactivity of phytomedicine.Asian Pac J Trop Biomed. 2014 May;4(Suppl 1):S1-7. doi: 10.12980/APJTB.4.2014C980. Asian Pac J Trop Biomed. 2014. PMID: 25183064 Free PMC article. Review.
References
-
- Devesa SS. The incidence of retinoblastoma. Am J Ophthalmol. 1975;80(2):263–265. - PubMed
-
- Bishop JO, Madson EC. Retinoblastoma. Review of the current status. Surv Ophthalmol. 1975;19(6):342–366. - PubMed
-
- Shields CL, Shields JA. Diagnosis and management of retinoblastoma. Cancer Control. 2004;11(5):317–327. - PubMed
-
- Abramson DH, Schefler AC. Update on retinoblastoma. Retina. 2004;24(6):828–848. - PubMed
-
- Byrne J, Fears TR, Whitney C, Parry DM. Survival after retinoblastoma: long-term consequences and family history of cancer. Med Pediatr Oncol. 1995;24(3):160–165. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

