Gap junction-mediated electrical transmission: regulatory mechanisms and plasticity
- PMID: 22659675
- PMCID: PMC3437247
- DOI: 10.1016/j.bbamem.2012.05.026
Gap junction-mediated electrical transmission: regulatory mechanisms and plasticity
Erratum in
- Biochim Biophys Acta. 2014 Mar;1838(3):1056
Abstract
The term synapse applies to cellular specializations that articulate the processing of information within neural circuits by providing a mechanism for the transfer of information between two different neurons. There are two main modalities of synaptic transmission: chemical and electrical. While most efforts have been dedicated to the understanding of the properties and modifiability of chemical transmission, less is still known regarding the plastic properties of electrical synapses, whose structural correlate is the gap junction. A wealth of data indicates that, rather than passive intercellular channels, electrical synapses are more dynamic and modifiable than was generally perceived. This article will discuss the factors determining the strength of electrical transmission and review current evidence demonstrating its dynamic properties. Like their chemical counterparts, electrical synapses can also be plastic and modifiable. This article is part of a Special Issue entitled: The Communicating junctions, roles and dysfunctions.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures
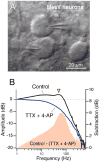
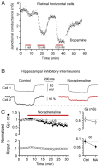
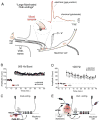

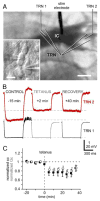
Similar articles
-
Dynamics of electrical transmission at club endings on the Mauthner cells.Brain Res Brain Res Rev. 2004 Dec;47(1-3):227-44. doi: 10.1016/j.brainresrev.2004.06.010. Brain Res Brain Res Rev. 2004. PMID: 15572174 Review.
-
The components of an electrical synapse as revealed by expansion microscopy of a single synaptic contact.Elife. 2024 Jul 12;13:e91931. doi: 10.7554/eLife.91931. Elife. 2024. PMID: 38994821 Free PMC article.
-
Electrical synapses in mammalian CNS: Past eras, present focus and future directions.Biochim Biophys Acta Biomembr. 2018 Jan;1860(1):102-123. doi: 10.1016/j.bbamem.2017.05.019. Epub 2017 Jun 1. Biochim Biophys Acta Biomembr. 2018. PMID: 28577972 Free PMC article. Review.
-
Plasticity of Retinal Gap Junctions: Roles in Synaptic Physiology and Disease.Annu Rev Vis Sci. 2018 Sep 15;4:79-100. doi: 10.1146/annurev-vision-091517-034133. Epub 2018 Jun 11. Annu Rev Vis Sci. 2018. PMID: 29889655 Review.
-
Connexin35 mediates electrical transmission at mixed synapses on Mauthner cells.J Neurosci. 2003 Aug 20;23(20):7489-503. doi: 10.1523/JNEUROSCI.23-20-07489.2003. J Neurosci. 2003. PMID: 12930787 Free PMC article.
Cited by
-
The role of gap junctions in cell death and neuromodulation in the retina.Neural Regen Res. 2021 Oct;16(10):1911-1920. doi: 10.4103/1673-5374.308069. Neural Regen Res. 2021. PMID: 33642359 Free PMC article. Review.
-
Gap junctional signaling in pattern regulation: Physiological network connectivity instructs growth and form.Dev Neurobiol. 2017 May;77(5):643-673. doi: 10.1002/dneu.22405. Epub 2016 Jun 24. Dev Neurobiol. 2017. PMID: 27265625 Free PMC article. Review.
-
Variability and directionality of inferior olive neuron dendrites revealed by detailed 3D characterization of an extensive morphological library.Brain Struct Funct. 2019 May;224(4):1677-1695. doi: 10.1007/s00429-019-01859-z. Epub 2019 Mar 30. Brain Struct Funct. 2019. PMID: 30929054 Free PMC article.
-
Re-membering the body: applications of computational neuroscience to the top-down control of regeneration of limbs and other complex organs.Integr Biol (Camb). 2015 Dec;7(12):1487-517. doi: 10.1039/c5ib00221d. Epub 2015 Nov 16. Integr Biol (Camb). 2015. PMID: 26571046 Free PMC article. Review.
-
On Having No Head: Cognition throughout Biological Systems.Front Psychol. 2016 Jun 21;7:902. doi: 10.3389/fpsyg.2016.00902. eCollection 2016. Front Psychol. 2016. PMID: 27445884 Free PMC article. Review.
References
-
- Bartelmez GW, Hoerr NL. The vestibular club endings in Ameiurus. Further evidence on the morphology of the synapse. J Comp Neurol. 1933;67:401–428.
-
- Pereda A, Faber DS. Physiology of the Mauthner Cell: Discovery and Properties. In: Farrell AP, editor. Encyclopedia of Fish Physiology: From Genome to Environment. San Diego: Academic Press; 2011.
-
- Faber DS, Pereda A. Physiology of the Mauthner cell: Functions. In: Farrell AP, editor. Encyclopedia of Fish Physiology: From Genome to Environment. San Diego: Academic Press; 2011. pp. 73–79.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

