Expression and purification of recombinant human tyrosine hydroxylase as a fusion protein in Escherichia coli
- PMID: 22659380
- PMCID: PMC3525113
- DOI: 10.1016/j.pep.2012.05.007
Expression and purification of recombinant human tyrosine hydroxylase as a fusion protein in Escherichia coli
Abstract
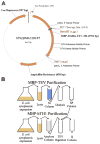
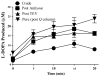
Tyrosine hydroxylase is the rate-limiting step in the synthesis of dopamine and is tightly regulated. Previous studies have shown it to be covalently modified and potently inhibited by 3,4-dihydroxyphenylacetaldehyde (DOPAL), an endogenous neurotoxin via dopamine catabolism which is relevant to Parkinson's disease. In order to elucidate the mechanism of enzyme inhibition, a source of pure, active tyrosine hydroxylase was necessary. The cloning and novel purification of human recombinant TH from Escherichia coli is described here. This procedure led to the recovery of ~23 mg of pure, active and stable enzyme exhibiting a specific activity of ~17 nmol/min/mg. The enzyme produced with this procedure can be used to delineate the tyrosine hydroxylase inhibition by DOPAL and its relationship to Parkinson's disease. This procedure improves upon previous methods because the fusion protein gives rise to high expression and convenient affinity-capture, and the cleaved and highly purified hTH makes the product useful for a wider variety of applications.
Copyright © 2012. Published by Elsevier Inc.
Figures

Similar articles
-
Cloning and characterization of a novel enzyme: tyrosine hydroxylase from Schistosoma japonicum.Parasitol Res. 2011 Oct;109(4):1065-74. doi: 10.1007/s00436-011-2347-y. Epub 2011 May 10. Parasitol Res. 2011. PMID: 21556690
-
Expression of mouse tyrosine hydroxylase in Escherichia coli.Biochem Biophys Res Commun. 1991 Jul 31;178(2):664-71. doi: 10.1016/0006-291x(91)90159-5. Biochem Biophys Res Commun. 1991. PMID: 1677565
-
Cloning and characterization of a novel form of tyrosine hydroxylase from the human parasite, Schistosoma mansoni.J Neurochem. 1998 Oct;71(4):1369-80. doi: 10.1046/j.1471-4159.1998.71041369.x. J Neurochem. 1998. PMID: 9751167
-
High level expression, purification and characterization of active fusion human C1q and tumor necrosis factor related protein 2 (hCTRP2) in Escherichia coli.Protein Expr Purif. 2011 Sep;79(1):1-6. doi: 10.1016/j.pep.2011.03.013. Epub 2011 Mar 29. Protein Expr Purif. 2011. PMID: 21453774 Review.
-
Current status of tyrosine hydroxylase in management of Parkinson's disease.CNS Neurol Disord Drug Targets. 2012 Jun 1;11(4):450-5. doi: 10.2174/187152712800792910. CNS Neurol Disord Drug Targets. 2012. PMID: 22583428 Review.
Cited by
-
Real-time monitoring of tyrosine hydroxylase activity using a plate reader assay.Anal Biochem. 2013 Jan 1;432(1):11-5. doi: 10.1016/j.ab.2012.09.005. Epub 2012 Sep 23. Anal Biochem. 2013. PMID: 23010244 Free PMC article.
-
Novel enhancement mechanism of tyrosine hydroxylase enzymatic activity by nitric oxide through S-nitrosylation.Sci Rep. 2017 Mar 13;7:44154. doi: 10.1038/srep44154. Sci Rep. 2017. PMID: 28287127 Free PMC article.
-
Challenges and opportunities in the purification of recombinant tagged proteins.Biotechnol Adv. 2014 Mar-Apr;32(2):366-81. doi: 10.1016/j.biotechadv.2013.12.001. Epub 2013 Dec 12. Biotechnol Adv. 2014. PMID: 24334194 Free PMC article. Review.
-
Stable preparations of tyrosine hydroxylase provide the solution structure of the full-length enzyme.Sci Rep. 2016 Jul 27;6:30390. doi: 10.1038/srep30390. Sci Rep. 2016. PMID: 27462005 Free PMC article.
-
Cellular localization of dieldrin and structure-activity relationship of dieldrin analogues in dopaminergic cells.Chem Res Toxicol. 2013 Jul 15;26(7):1043-54. doi: 10.1021/tx300458b. Epub 2013 Jun 27. Chem Res Toxicol. 2013. PMID: 23763672 Free PMC article.
References
-
- Elsworth JD, Roth RH. Dopamine synthesis, uptake, metabolism, and receptors: relevance to gene therapy of Parkinson’s disease. Exp Neurol. 1997;144:4–9. - PubMed
-
- Nagatsu T, Levitt M, Udenfriend S. Tyrosine hydroxylase. The Initial step in norepinephrine biosynthesis. J Biol Chem. 1964;239:2910–2917. - PubMed
-
- Daubner SC, Lauriano C, Haycock JW, Fitzpatrick PF. Site-directed mutagenesis of serine 40 of rat tyrosine hydroxylase. Effects of dopamine and cAMP-dependent phosphorylation on enzyme activity. J Biol Chem. 1992;267:12639–12646. - PubMed
-
- Xu Y, Stokes AH, Roskoski R, Jr, Vrana KE. Dopamine, in the presence of tyrosinase, covalently modifies and inactivates tyrosine hydroxylase. J Neurosci Res. 1998;54:691–697. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

