Genetically separable functions of the MEC-17 tubulin acetyltransferase affect microtubule organization
- PMID: 22658602
- PMCID: PMC3382010
- DOI: 10.1016/j.cub.2012.03.066
Genetically separable functions of the MEC-17 tubulin acetyltransferase affect microtubule organization
Abstract
Background: Microtubules (MTs) are formed from the lateral association of 11-16 protofilament chains of tubulin dimers, with most cells containing 13-protofilament (13-p) MTs. How these different MTs are formed is unknown, although the number of protofilaments may depend on the nature of the α- and β-tubulins.
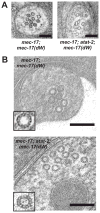
Results: Here we show that the enzymatic activity of the Caenorhabiditis elegans α-tubulin acetyltransferase (α-TAT) MEC-17 allows the production of 15-p MTs in the touch receptor neurons (TRNs) MTs. Without MEC-17, MTs with between 11 and 15 protofilaments are seen. Loss of this enzymatic activity also changes the number and organization of the TRN MTs and affects TRN axonal morphology. In contrast, enzymatically inactive MEC-17 is sufficient for touch sensitivity and proper process outgrowth without correcting the MT defects. Thus, in addition to demonstrating that MEC-17 is required for MT structure and organization, our results suggest that the large number of 15-p MTs, normally found in the TRNs, is not essential for mechanosensation.
Conclusion: These experiments reveal a specific role for α-TAT in the formation of MTs and in the production of higher order MTs arrays. In addition, our results indicate that the α-TAT protein has functions that require acetyltransferase activity (such as the determination of protofilament number) and others that do not (presence of internal MT structures).
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
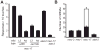




Comment in
-
Microtubules: MEC-17 moonlights in the lumen.Curr Biol. 2012 Jun 19;22(12):R483-5. doi: 10.1016/j.cub.2012.05.027. Curr Biol. 2012. PMID: 22720680
Similar articles
-
Posttranslational acetylation of α-tubulin constrains protofilament number in native microtubules.Curr Biol. 2012 Jun 19;22(12):1066-74. doi: 10.1016/j.cub.2012.05.012. Epub 2012 May 31. Curr Biol. 2012. PMID: 22658592 Free PMC article.
-
Microtubules: MEC-17 moonlights in the lumen.Curr Biol. 2012 Jun 19;22(12):R483-5. doi: 10.1016/j.cub.2012.05.027. Curr Biol. 2012. PMID: 22720680
-
The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation.Proc Natl Acad Sci U S A. 2010 Dec 14;107(50):21517-22. doi: 10.1073/pnas.1013728107. Epub 2010 Nov 10. Proc Natl Acad Sci U S A. 2010. PMID: 21068373 Free PMC article.
-
Functions of the tubulin code in the C. elegans nervous system.Mol Cell Neurosci. 2022 Dec;123:103790. doi: 10.1016/j.mcn.2022.103790. Epub 2022 Nov 9. Mol Cell Neurosci. 2022. PMID: 36368428 Review.
-
Tubulins in C. elegans.WormBook. 2018 Aug 4;2018:1-32. doi: 10.1895/wormbook.1.182.1. WormBook. 2018. PMID: 29381886 Free PMC article. Review.
Cited by
-
Tubulin acetylation: responsible enzymes, biological functions and human diseases.Cell Mol Life Sci. 2015 Nov;72(22):4237-55. doi: 10.1007/s00018-015-2000-5. Epub 2015 Jul 31. Cell Mol Life Sci. 2015. PMID: 26227334 Free PMC article. Review.
-
Alpha-tubulin acetyltransferase/MEC-17 regulates cancer cell migration and invasion through epithelial-mesenchymal transition suppression and cell polarity disruption.Sci Rep. 2018 Nov 30;8(1):17477. doi: 10.1038/s41598-018-35392-6. Sci Rep. 2018. PMID: 30504808 Free PMC article.
-
Tubulin acetylation protects long-lived microtubules against mechanical ageing.Nat Cell Biol. 2017 Apr;19(4):391-398. doi: 10.1038/ncb3481. Epub 2017 Feb 27. Nat Cell Biol. 2017. PMID: 28250419 Free PMC article.
-
Sperm Associated Antigen 6 (SPAG6) Regulates Fibroblast Cell Growth, Morphology, Migration and Ciliogenesis.Sci Rep. 2015 Nov 20;5:16506. doi: 10.1038/srep16506. Sci Rep. 2015. PMID: 26585507 Free PMC article.
-
C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress.Nature. 2017 Feb 16;542(7641):367-371. doi: 10.1038/nature21362. Epub 2017 Feb 8. Nature. 2017. PMID: 28178240 Free PMC article.
References
-
- Burton PR, Hinkley RE. Further electron miscroscopic characterization of axoplasmic microtubules of the ventral cord of the crayfish. J Submicrosc Cytol. 1974;6:311–326.
-
- Afzelius BA, Bellon PL, Lanzavecchia S. Microtubules and their protofilaments in the flagellum of an insect spermatozoon. J Cell Sci. 1990;95(Pt 2):207–217. - PubMed
-
- Hirose K, Amos WB, Lockhart A, Cross RA, Amos LA. Three-dimensional cryoelectron microscopy of 16-protofilament microtubules: structure, polarity, and interaction with motor proteins. J Struct Biol. 1997;118:140–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

