Novel on-demand droplet generation for selective fluid sample extraction
- PMID: 22655015
- PMCID: PMC3360719
- DOI: 10.1063/1.3699972
Novel on-demand droplet generation for selective fluid sample extraction
Abstract
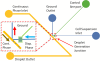
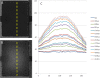
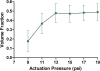
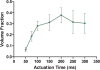
A novel microfluidic device enabling selective generation of droplets and encapsulation of targets is presented. Unlike conventional methods, the presented mechanism generates droplets with unique selectivity by utilizing a K-junction design. The K-junction is a modified version of the classic T-junction with an added leg that serves as the exit channel for waste. The dispersed phase fluid enters from one diagonal of the K and exits the other diagonal while the continuous phase travels in the straight leg of the K. The intersection forms an interface that allows the dispersed phase to be controllably injected through actuation of an elastomer membrane located above the inlet channel near the interface. We have characterized two critical components in controlling the droplet size-membrane actuation pressure and timing as well as identified the region of fluid in which the droplet will be formed. This scheme will have applications in fluid sampling processes and selective encapsulation of materials. Selective encapsulation of a single cell from the dispersed phase fluid is demonstrated as an example of functionality of this design.
Figures




Similar articles
-
Versatile on-demand droplet generation for controlled encapsulation.Biomicrofluidics. 2014 Jun 12;8(3):034112. doi: 10.1063/1.4874715. eCollection 2014 May. Biomicrofluidics. 2014. PMID: 25379072 Free PMC article.
-
Droplet Microfluidics in Thermoplastics: Device Fabrication, Droplet Generation, and Content Manipulation using Integrated Electric and Magnetic Fields.Anal Methods. 2018 Sep 21;10(35):4264-4274. doi: 10.1039/C8AY01474D. Epub 2018 Aug 20. Anal Methods. 2018. PMID: 30886651 Free PMC article.
-
A simple capillary-based open microfluidic device for size on-demand high-throughput droplet/bubble/microcapsule generation.Lab Chip. 2018 Sep 11;18(18):2806-2815. doi: 10.1039/c8lc00479j. Lab Chip. 2018. PMID: 30112532
-
Block-and-break generation of microdroplets with fixed volume.Biomicrofluidics. 2013 Apr 10;7(2):24108. doi: 10.1063/1.4801637. eCollection 2013. Biomicrofluidics. 2013. PMID: 24404013 Free PMC article.
-
Passive and active droplet generation with microfluidics: a review.Lab Chip. 2016 Dec 20;17(1):34-75. doi: 10.1039/c6lc01018k. Lab Chip. 2016. PMID: 27841886 Review.
Cited by
-
Active Flow Control and Dynamic Analysis in Droplet Microfluidics.Annu Rev Anal Chem (Palo Alto Calif). 2021 Jul 27;14(1):133-153. doi: 10.1146/annurev-anchem-122120-042627. Annu Rev Anal Chem (Palo Alto Calif). 2021. PMID: 33979546 Free PMC article. Review.
-
On demand nanoliter-scale microfluidic droplet generation, injection, and mixing using a passive microfluidic device.Biomicrofluidics. 2015 Feb 12;9(1):014119. doi: 10.1063/1.4907895. eCollection 2015 Jan. Biomicrofluidics. 2015. PMID: 25759752 Free PMC article.
-
On-demand generation and mixing of liquid-in-gas slugs with digitally-programmable composition and size.J Micromech Microeng. 2015 Aug;25(8):084006. doi: 10.1088/0960-1317/25/8/084006. Epub 2015 Jul 22. J Micromech Microeng. 2015. PMID: 29167603 Free PMC article.
References
-
- Piotr G., Irina G., Willow D., George M. W., Eugenia K., and Howard A. S., Appl. Phys. Lett. 85(13 ), 2649–2651 (2004).10.1063/1.1796526 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
