Inborn errors of human STAT1: allelic heterogeneity governs the diversity of immunological and infectious phenotypes
- PMID: 22651901
- PMCID: PMC3477860
- DOI: 10.1016/j.coi.2012.04.011
Inborn errors of human STAT1: allelic heterogeneity governs the diversity of immunological and infectious phenotypes
Abstract
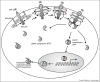
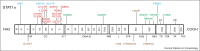
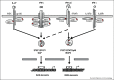
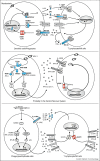
The genetic dissection of various human infectious diseases has led to the definition of inborn errors of human STAT1 immunity of four types, including (i) autosomal recessive (AR) complete STAT1 deficiency, (ii) AR partial STAT1 deficiency, (iii) autosomal dominant (AD) STAT1 deficiency, and (iv) AD gain of STAT1 activity. The two types of AR STAT1 defect give rise to a broad infectious phenotype with susceptibility to intramacrophagic bacteria (mostly mycobacteria) and viruses (herpes viruses at least), due principally to the impairment of IFN-γ-mediated and IFN-α/β-mediated immunity, respectively. Clinical outcome depends on the extent to which the STAT1 defect decreases responsiveness to these cytokines. AD STAT1 deficiency selectively predisposes individuals to mycobacterial disease, owing to the impairment of IFN-γ-mediated immunity, as IFN-α/β-mediated immunity is maintained. Finally, AD gain of STAT1 activity is associated with autoimmunity, probably owing to an enhancement of IFN-α/β-mediated immunity. More surprisingly, it is also associated with chronic mucocutaneous candidiasis, through as yet undetermined mechanisms involving an inhibition of the development of IL-17-producing T cells. Thus, germline mutations in human STAT1 define four distinct clinical disorders. Various combinations of viral, mycobacterial and fungal infections are therefore allelic at the human STAT1 locus. These experiments of Nature neatly highlight the clinical and immunological impact of the human genetic dissection of infectious phenotypes.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-γ immunity.Semin Immunol. 2014 Dec;26(6):454-70. doi: 10.1016/j.smim.2014.09.008. Epub 2014 Oct 26. Semin Immunol. 2014. PMID: 25453225 Free PMC article. Review.
-
Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis.J Exp Med. 2011 Aug 1;208(8):1635-48. doi: 10.1084/jem.20110958. Epub 2011 Jul 4. J Exp Med. 2011. PMID: 21727188 Free PMC article.
-
Inborn errors of STAT1 immunity.Curr Opin Immunol. 2021 Oct;72:59-64. doi: 10.1016/j.coi.2021.02.009. Epub 2021 Apr 8. Curr Opin Immunol. 2021. PMID: 33839590 Review.
-
Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-alpha/beta, IFN-gamma, and IFN-lambda in host defense.Immunol Rev. 2008 Dec;226:29-40. doi: 10.1111/j.1600-065X.2008.00698.x. Immunol Rev. 2008. PMID: 19161414 Review.
-
Genetic and Functional Identifying of Novel STAT1 Loss-of-Function Mutations in Patients with Diverse Clinical Phenotypes.J Clin Immunol. 2022 Nov;42(8):1778-1794. doi: 10.1007/s10875-022-01339-w. Epub 2022 Aug 17. J Clin Immunol. 2022. PMID: 35976469
Cited by
-
STAT1 Isoforms Differentially Regulate NK Cell Maturation and Anti-tumor Activity.Front Immunol. 2020 Sep 11;11:2189. doi: 10.3389/fimmu.2020.02189. eCollection 2020. Front Immunol. 2020. PMID: 33042133 Free PMC article.
-
Primary Immunodeficiencies With Defects in Innate Immunity: Focus on Orofacial Manifestations.Front Immunol. 2020 Jun 18;11:1065. doi: 10.3389/fimmu.2020.01065. eCollection 2020. Front Immunol. 2020. PMID: 32625202 Free PMC article. Review.
-
STAT1 deficiency predisposes to spontaneous otitis media.PLoS One. 2020 Sep 29;15(9):e0239952. doi: 10.1371/journal.pone.0239952. eCollection 2020. PLoS One. 2020. PMID: 32991625 Free PMC article.
-
Beyond monogenetic rare variants: tackling the low rate of genetic diagnoses in predominantly antibody deficiency.Cell Mol Immunol. 2021 Mar;18(3):588-603. doi: 10.1038/s41423-020-00520-8. Epub 2020 Aug 17. Cell Mol Immunol. 2021. PMID: 32801365 Free PMC article. Review.
-
Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis.Curr Opin Allergy Clin Immunol. 2012 Dec;12(6):616-22. doi: 10.1097/ACI.0b013e328358cc0b. Curr Opin Allergy Clin Immunol. 2012. PMID: 23026768 Free PMC article. Review.
References
-
- Levy D.E., Kessler D.S., Pine R., Darnell J.E., Jr. Cytoplasmic activation of ISGF3, the positive regulator of interferon-alpha-stimulated transcription, reconstituted in vitro. Genes Dev. 1989;3:1362–1371. - PubMed
-
- Darnell J.E., Jr., Kerr I.M., Stark G.R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

