Maternal smoking and the retinoid pathway in the developing lung
- PMID: 22651576
- PMCID: PMC3479035
- DOI: 10.1186/1465-9921-13-42
Maternal smoking and the retinoid pathway in the developing lung
Abstract
Background: Maternal smoking is a risk factor for pediatric lung disease, including asthma. Animal models suggest that maternal smoking causes defective alveolarization in the offspring. Retinoic acid signaling modulates both lung development and postnatal immune function. Thus, abnormalities in this pathway could mediate maternal smoking effects. We tested whether maternal smoking disrupts retinoic acid pathway expression and functioning in a murine model.
Methods: Female C57Bl/6 mice with/without mainstream cigarette smoke exposure (3 research cigarettes a day, 5 days a week) were mated to nonsmoking males. Cigarette smoke exposure continued throughout the pregnancy and after parturition. Lung tissue from the offspring was examined by mean linear intercept analysis and by quantitative PCR. Cell culture experiments using the type II cell-like cell line, A549, tested whether lipid-soluble cigarette smoke components affected binding and activation of retinoic acid response elements in vitro.
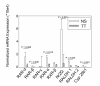
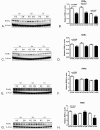
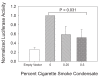
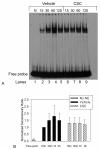
Results: Compared to tobacco-naïve mice, juvenile mice with tobacco toxin exposure had significantly (P < 0.05) increased mean linear intercepts, consistent with an alveolarization defect. Tobacco toxin exposure significantly (P < 0.05) decreased mRNA and protein expression of retinoic acid signaling pathway elements, including retinoic acid receptor alpha and retinoic acid receptor beta, with the greatest number of changes observed between postnatal days 3-5. Lipid-soluble cigarette smoke components significantly (P < 0.05) decreased retinoic acid-induced binding and activation of the retinoic acid receptor response element in A549 cells.
Conclusions: A murine model of maternal cigarette smoking causes abnormal alveolarization in association with altered retinoic acid pathway element expression in the offspring. An in vitro cell culture model shows that lipid-soluble components of cigarette smoke decrease retinoic acid response element activation. It is feasible that disruption of retinoic acid signaling contributes to the pediatric lung dysfunction caused by maternal smoking.
Figures


Similar articles
-
Damaging legacy: maternal cigarette smoking has long-term consequences for male offspring fertility.Hum Reprod. 2014 Dec;29(12):2719-35. doi: 10.1093/humrep/deu235. Epub 2014 Sep 30. Hum Reprod. 2014. PMID: 25269568
-
Effect of long-term maternal smoking on the offspring's lung health.Am J Physiol Lung Cell Mol Physiol. 2017 Aug 1;313(2):L416-L423. doi: 10.1152/ajplung.00134.2017. Epub 2017 May 18. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28522560
-
Uteroplacental Insufficiency Alters the Retinoid Pathway and Lung Development in Newborn Rats.Pediatr Neonatol. 2016 Dec;57(6):508-514. doi: 10.1016/j.pedneo.2016.03.003. Epub 2016 Apr 1. Pediatr Neonatol. 2016. PMID: 27118112
-
Pulmonary Effects of Maternal Smoking on the Fetus and Child: Effects on Lung Development, Respiratory Morbidities, and Life Long Lung Health.Paediatr Respir Rev. 2017 Jan;21:27-33. doi: 10.1016/j.prrv.2016.08.005. Epub 2016 Aug 19. Paediatr Respir Rev. 2017. PMID: 27639458 Free PMC article. Review.
-
Life-long programming implications of exposure to tobacco smoking and nicotine before and soon after birth: evidence for altered lung development.Int J Environ Res Public Health. 2011 Mar;8(3):875-98. doi: 10.3390/ijerph8030875. Epub 2011 Mar 16. Int J Environ Res Public Health. 2011. PMID: 21556184 Free PMC article. Review.
Cited by
-
Inter- and transgenerational epigenetic inheritance: evidence in asthma and COPD?Clin Epigenetics. 2015 May 1;7(1):53. doi: 10.1186/s13148-015-0085-1. eCollection 2015. Clin Epigenetics. 2015. PMID: 26052354 Free PMC article.
-
N-Acetyltransferase 2 Genotypes Are Associated With Diisocyanate-Induced Asthma.J Occup Environ Med. 2015 Dec;57(12):1331-6. doi: 10.1097/JOM.0000000000000561. J Occup Environ Med. 2015. PMID: 26641831 Free PMC article.
-
Air pollution as an early determinant of COPD.Eur Respir Rev. 2022 Aug 10;31(165):220059. doi: 10.1183/16000617.0059-2022. Print 2022 Sep 30. Eur Respir Rev. 2022. PMID: 35948393 Free PMC article. Review.
-
Proteomic differences with and without ozone-exposure in a smoking-induced emphysema lung model.Korean J Intern Med. 2015 Jan;30(1):62-72. doi: 10.3904/kjim.2015.30.1.62. Epub 2014 Dec 30. Korean J Intern Med. 2015. PMID: 25589837 Free PMC article.
-
Maternal smoke exposure decreases mesenchymal proliferation and modulates Rho-GTPase-dependent actin cytoskeletal signaling in fetal lungs.FASEB J. 2017 Jun;31(6):2340-2351. doi: 10.1096/fj.201601063R. Epub 2017 Feb 16. FASEB J. 2017. PMID: 28209772 Free PMC article.
References
-
- Tager IB, Hanrahan JP, Tosteson TD, Castile RG, Brown RW, Weiss ST, Speizer FE. Lung function, pre- and post-natal smoke exposure, and wheezing in the first year of life. Am Rev Respir Dis. 1993;147:811–817. - PubMed
-
- Tager IB, Ngo L, Hanrahan JP. Maternal smoking during pregnancy: effects on lung function during the first 18 months of life. Am J Respir Crit Care Med. 1995;152:977–983. - PubMed
-
- Martinez FD, Cline M, Burrows B. Increased incidence of asthma in children of smoking mothers. Pediatrics. 1992;89:21–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

