Calcyon, a mammalian specific NEEP21 family member, interacts with adaptor protein complex 3 (AP-3) and regulates targeting of AP-3 cargoes
- PMID: 22650988
- PMCID: PMC3813962
- DOI: 10.1111/j.1471-4159.2012.07814.x
Calcyon, a mammalian specific NEEP21 family member, interacts with adaptor protein complex 3 (AP-3) and regulates targeting of AP-3 cargoes
Abstract
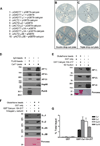
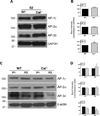
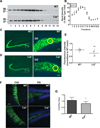
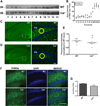
Calcyon is a neural enriched, single transmembrane protein that interacts with clathrin light chain and stimulates clathrin assembly and clathrin-mediated endocytosis. A similar property is shared by the heterotetrameric adaptor protein (AP) complexes AP-1, AP-2, and AP-3 which recruit cargoes for insertion into clathrin coated transport vesicles. Here we report that AP medium (μ) subunits interact with a YXXØ-type tyrosine motif located at residues 133-136 in the cytoplasmic domain of calcyon. Site specific mutagenesis of the critical tyrosine and bulky hydrophobic residues tyrosine 133 and methionine 136 preferentially abrogated binding of the ubiquitous and neuronal isoforms of μ3, and also impacted μ1 and μ2 binding to a lesser degree. The relevance of these interactions was explored in vivo using mice harboring null alleles of calcyon. As seen in the mutagenesis studies, calcyon deletion in mice preferentially altered the subcellular distribution of AP-3 suggesting that calcyon could regulate membrane-bound pools of AP-3 and AP-3 function. To test this hypothesis, we focused on the hilar region of hippocampus, where levels of calcyon, AP-3, and AP-3 cargoes are abundant. We analyzed brain cryosections from control and calcyon null mice for zinc transporter 3 (ZnT3), and phosphatidylinositol-4-kinase type II alpha (PI4KIIα), two well-defined AP-3 cargoes. Confocal microscopy indicated that ZnT3 and PI4KIIα are significantly reduced in the hippocampal mossy fibers of calcyon knock-out brain, a phenotype previously described in AP-3 deficiencies. Altogether, our data suggest that calcyon directly interacts with μ3A and μ3B, and regulates the subcellular distribution of AP-3 and the targeting of AP-3 cargoes.
© 2012 The Authors. Journal of Neurochemistry © 2012 International Society for Neurochemistry.
Figures


Similar articles
-
Calcyon, a novel partner of clathrin light chain, stimulates clathrin-mediated endocytosis.J Biol Chem. 2006 Jun 2;281(22):15182-93. doi: 10.1074/jbc.M600265200. Epub 2006 Apr 4. J Biol Chem. 2006. PMID: 16595675
-
Phosphorylation of the medium chain subunit of the AP-2 adaptor complex does not influence its interaction with the tyrosine based internalisation motif of TGN38.FEBS Lett. 1999 Feb 12;444(2-3):195-200. doi: 10.1016/s0014-5793(99)00066-6. FEBS Lett. 1999. PMID: 10050758
-
Structural basis for the recognition of tyrosine-based sorting signals by the μ3A subunit of the AP-3 adaptor complex.J Biol Chem. 2013 Mar 29;288(13):9563-71. doi: 10.1074/jbc.M113.450775. Epub 2013 Feb 12. J Biol Chem. 2013. PMID: 23404500 Free PMC article.
-
Complementary roles of the neuron-enriched endosomal proteins NEEP21 and calcyon in neuronal vesicle trafficking.J Neurochem. 2015 Jan;132(1):20-31. doi: 10.1111/jnc.12989. J Neurochem. 2015. PMID: 25376768 Review.
-
Clathrin adaptors really adapt.Cell. 2002 May 17;109(4):413-6. doi: 10.1016/s0092-8674(02)00751-1. Cell. 2002. PMID: 12086597 Review.
Cited by
-
Coupling of microtubule motors with AP-3 generated organelles in axons by NEEP21 family member calcyon.Mol Biol Cell. 2018 Aug 15;29(17):2055-2068. doi: 10.1091/mbc.E18-01-0007. Epub 2018 Jun 27. Mol Biol Cell. 2018. PMID: 29949458 Free PMC article.
-
Calcyon stimulates neuregulin 1 maturation and signaling.Mol Psychiatry. 2015 Oct;20(10):1251-60. doi: 10.1038/mp.2014.131. Epub 2014 Oct 28. Mol Psychiatry. 2015. PMID: 25349163
-
AP-3 adaptor complex-mediated vesicle trafficking.Biophys Rep. 2021 Apr 30;7(2):91-100. doi: 10.52601/bpr.2021.200051. Biophys Rep. 2021. PMID: 37288146 Free PMC article.
-
The effect of psychoactive bacteria, Bifidobacterium longum Rosell®-175 and Lactobacillus rhamnosus JB-1, on brain proteome profiles in mice.Psychopharmacology (Berl). 2024 May;241(5):925-945. doi: 10.1007/s00213-023-06519-z. Epub 2023 Dec 29. Psychopharmacology (Berl). 2024. PMID: 38156998 Free PMC article.
-
Knockout of AMPA receptor binding protein Neuron-Specific Gene 2 (NSG2) enhances associative learning and cognitive flexibility.Res Sq [Preprint]. 2024 Aug 27:rs.3.rs-4790348. doi: 10.21203/rs.3.rs-4790348/v1. Res Sq. 2024. Update in: Mol Brain. 2024 Dec 18;17(1):95. doi: 10.1186/s13041-024-01158-7 PMID: 39257983 Free PMC article. Updated. Preprint.
References
-
- Ali MK, Bergson C. Elevated intracellular calcium triggers recruitment of the receptor cross-talk accessory protein calcyon to the plasma membrane. J.Biol.Chem. 2003;278:51654–51663. - PubMed
-
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. - PubMed
-
- Chicurel ME, Harris KM. Three-dimensional analysis of the structure and composition of CA3 branched dendritic spines and their synaptic relationships with mossy fiber boutons in the rat hippocampus. J. Comp. Neurol. 1992;325:169–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

