Modern Technologies for Creating Synthetic Antibodies for Clinical application
- PMID: 22649585
- PMCID: PMC3347500
Modern Technologies for Creating Synthetic Antibodies for Clinical application
Abstract
The modular structure and versatility of antibodies enables one to modify natural immunoglobulins in different ways for various clinical applications. Rational design and molecular engineering make it possible to directionally modify the molecular size, affinity, specificity, and immunogenicity and effector functions of an antibody, as well as to combine them with other functional agents. This review focuses on up-to-date methods of antibody engineering for diagnosing and treating various diseases, particularly on new technologies meant to refine the effector functions of therapeutic antibodies.
Figures
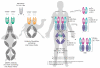
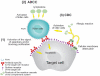
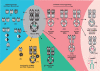
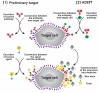

Similar articles
-
Engineering antibodies for clinical applications.Trends Biotechnol. 2007 Jul;25(7):307-16. doi: 10.1016/j.tibtech.2007.05.001. Epub 2007 May 21. Trends Biotechnol. 2007. PMID: 17512622 Review.
-
Overview on the use of therapeutic antibodies in drug discovery.Curr Protoc Pharmacol. 2005 Jan 1;Chapter 9:Unit 9.7. doi: 10.1002/0471141755.ph0907s27. Curr Protoc Pharmacol. 2005. PMID: 22294129
-
The biotechnology and applications of antibody engineering.Mol Biotechnol. 1995 Apr;3(2):139-54. doi: 10.1007/BF02789110. Mol Biotechnol. 1995. PMID: 7620975 Review.
-
Monoclonal antibodies: technologies for early discovery and engineering.Crit Rev Biotechnol. 2018 May;38(3):394-408. doi: 10.1080/07388551.2017.1357002. Epub 2017 Aug 8. Crit Rev Biotechnol. 2018. PMID: 28789584 Review.
-
Antibody Engineering.Microbiol Spectr. 2014 Feb;2(1):AID-0007-2012. doi: 10.1128/microbiolspec.AID-0007-12. Microbiol Spectr. 2014. PMID: 26082127 Review.
Cited by
-
Molecularly Imprinted Ligand-Free Nanogels for Recognizing Bee Venom-Originated Phospholipase A2 Enzyme.Polymers (Basel). 2022 Oct 7;14(19):4200. doi: 10.3390/polym14194200. Polymers (Basel). 2022. PMID: 36236149 Free PMC article.
-
Phage display on the base of filamentous bacteriophages: application for recombinant antibodies selection.Acta Naturae. 2009 Oct;1(3):20-8. Acta Naturae. 2009. PMID: 22649612 Free PMC article.
-
Minibody-Based and scFv-Based Antibody Fragment-Drug Conjugates Selectively Eliminate GD2-Positive Tumor Cells.Int J Mol Sci. 2023 Jan 8;24(2):1239. doi: 10.3390/ijms24021239. Int J Mol Sci. 2023. PMID: 36674755 Free PMC article.
-
Immunoglobulin A, an Active Liaison for Host-Microbiota Homeostasis.Microorganisms. 2021 Oct 8;9(10):2117. doi: 10.3390/microorganisms9102117. Microorganisms. 2021. PMID: 34683438 Free PMC article. Review.
-
Broadly Neutralizing Antibodies against HIV-1 As a Novel Aspect of the Immune Response.Acta Naturae. 2015 Oct-Dec;7(4):11-21. Acta Naturae. 2015. PMID: 26798488 Free PMC article.
References
-
- Erhlich P. Physiology or medicine 1901-1921. Amsterdam: Elsevier Publishing Co.; 1967. pp. 304–320.
-
- Desmyter A, Decanniere K, Muyldermans S. et al. J. Biol. Chem. 2001;276:26285–26290. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
