Enhanced influenza virus-like particle vaccines containing the extracellular domain of matrix protein 2 and a Toll-like receptor ligand
- PMID: 22647270
- PMCID: PMC3416094
- DOI: 10.1128/CVI.00153-12
Enhanced influenza virus-like particle vaccines containing the extracellular domain of matrix protein 2 and a Toll-like receptor ligand
Abstract
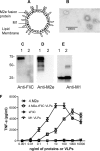
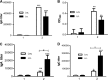
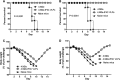
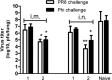
The extracellular domain of matrix protein 2 (M2e) is conserved among influenza A viruses. The goal of this project is to develop enhanced influenza vaccines with broad protective efficacy using the M2e antigen. We designed a membrane-anchored fusion protein by replacing the hyperimmunogenic region of Salmonella enterica serovar Typhimurium flagellin (FliC) with four repeats of M2e (4.M2e-tFliC) and fusing it to a membrane anchor from influenza virus hemagglutinin (HA). The fusion protein was incorporated into influenza virus M1-based virus-like particles (VLPs). These VLPs retained Toll-like receptor 5 (TLR5) agonist activity comparable to that of soluble FliC. Mice immunized with the VLPs by either intramuscular or intranasal immunization showed high levels of systemic M2-specific antibody responses compared to the responses to soluble 4.M2e protein. High mucosal antibody titers were also induced in intranasally immunized mice. All intranasally immunized mice survived lethal challenges with live virus, while intramuscularly immunized mice showed only partial protection, revealing better protection by the intranasal route. These results indicate that a combination of M2e antigens and TLR ligand adjuvants in VLPs has potential for development of a broadly protective influenza A virus vaccine.
Figures


Similar articles
-
Protein nanoparticle vaccine based on flagellin carrier fused to influenza conserved epitopes confers full protection against influenza A virus challenge.Virology. 2017 Sep;509:82-89. doi: 10.1016/j.virol.2017.06.001. Epub 2017 Jun 13. Virology. 2017. PMID: 28622575 Free PMC article.
-
Potent immunogenicity and efficacy of a universal influenza vaccine candidate comprising a recombinant fusion protein linking influenza M2e to the TLR5 ligand flagellin.Vaccine. 2008 Jan 10;26(2):201-14. doi: 10.1016/j.vaccine.2007.10.062. Epub 2007 Nov 20. Vaccine. 2008. PMID: 18063235
-
Intranasal adenovirus-vectored vaccine for induction of long-lasting humoral immunity-mediated broad protection against influenza in mice.J Virol. 2014 Sep 1;88(17):9693-703. doi: 10.1128/JVI.00823-14. Epub 2014 Jun 11. J Virol. 2014. PMID: 24920793 Free PMC article.
-
Inclusion of membrane-anchored LTB or flagellin protein in H5N1 virus-like particles enhances protective responses following intramuscular and oral immunization of mice.Vaccine. 2018 Sep 25;36(40):5990-5998. doi: 10.1016/j.vaccine.2018.08.053. Epub 2018 Aug 30. Vaccine. 2018. PMID: 30172635
-
Flagellin a toll-like receptor 5 agonist as an adjuvant in chicken vaccines.Clin Vaccine Immunol. 2014 Mar;21(3):261-70. doi: 10.1128/CVI.00669-13. Epub 2014 Jan 22. Clin Vaccine Immunol. 2014. PMID: 24451328 Free PMC article. Review.
Cited by
-
Virus-like particles containing the tetrameric ectodomain of influenza matrix protein 2 and flagellin induce heterosubtypic protection in mice.Biomed Res Int. 2013;2013:686549. doi: 10.1155/2013/686549. Epub 2013 Jul 30. Biomed Res Int. 2013. PMID: 23984396 Free PMC article.
-
Protein nanoparticle vaccine based on flagellin carrier fused to influenza conserved epitopes confers full protection against influenza A virus challenge.Virology. 2017 Sep;509:82-89. doi: 10.1016/j.virol.2017.06.001. Epub 2017 Jun 13. Virology. 2017. PMID: 28622575 Free PMC article.
-
Universal influenza vaccines: from viruses to nanoparticles.Expert Rev Vaccines. 2018 Nov;17(11):967-976. doi: 10.1080/14760584.2018.1541408. Epub 2018 Nov 2. Expert Rev Vaccines. 2018. PMID: 30365905 Free PMC article. Review.
-
The minimalist architectures of viroporins and their therapeutic implications.Biochim Biophys Acta. 2014 Apr;1838(4):1058-67. doi: 10.1016/j.bbamem.2013.09.004. Epub 2013 Sep 18. Biochim Biophys Acta. 2014. PMID: 24055819 Free PMC article. Review.
-
Immune responses to oral and IM administration of M2e-Hsp70 construct.Vet Res Commun. 2014 Jun;38(2):157-63. doi: 10.1007/s11259-014-9599-9. Epub 2014 Mar 5. Vet Res Commun. 2014. PMID: 24595641
References
-
- Ben-Yedidia T, Arnon R. 1998. Effect of pre-existing carrier immunity on the efficacy of synthetic influenza vaccine. Immunol. Lett. 64:9–15 - PubMed
-
- Black RA, Rota PA, Gorodkova N, Klenk HD, Kendal AP. 1993. Antibody response to the M2 protein of influenza A virus expressed in insect cells. J. Gen. Virol. 74(Pt 1):143–146 - PubMed
-
- Brandtzaeg P. 2003. Role of mucosal immunity in influenza. Dev. Biol. (Basel) 115:39–48 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

