Cell-based therapy for prevention and reversal of myocardial remodeling
- PMID: 22636682
- PMCID: PMC3423164
- DOI: 10.1152/ajpheart.00221.2012
Cell-based therapy for prevention and reversal of myocardial remodeling
Abstract
Although pharmacological and interventional advances have reduced the morbidity and mortality of ischemic heart disease, there is an ongoing need for novel therapeutic strategies that prevent or reverse progressive ventricular remodeling following myocardial infarction, the process that forms the substrate for ventricular failure. The development of cell-based therapy as a strategy to repair or regenerate injured tissue offers extraordinary promise for a powerful anti-remodeling therapy. In this regard, the field of cell therapy has made major advancements in the past decade. Accumulating data from preclinical studies have provided novel insights into stem cell engraftment, differentiation, and interactions with host cellular elements, as well as the effectiveness of various methods of cell delivery and accuracy of diverse imaging modalities to assess therapeutic efficacy. These findings have in turn guided rationally designed translational clinical investigations. Collectively, there is a growing understanding of the parameters that underlie successful cell-based approaches for improving heart structure and function in ischemic and other cardiomyopathies.
Figures

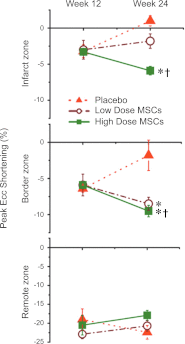
Similar articles
-
Cell therapy for cardiovascular disease: a comparison of methods of delivery.J Cardiovasc Transl Res. 2011 Apr;4(2):177-81. doi: 10.1007/s12265-010-9253-z. Epub 2010 Dec 23. J Cardiovasc Transl Res. 2011. PMID: 21181320 Free PMC article. Review.
-
Translational development of mesenchymal stem cell therapy for cardiovascular diseases.Tex Heart Inst J. 2009;36(2):145-7. Tex Heart Inst J. 2009. PMID: 19436809 Free PMC article. No abstract available.
-
ESC Working Group on Cellular Biology of the Heart: position paper for Cardiovascular Research: tissue engineering strategies combined with cell therapies for cardiac repair in ischaemic heart disease and heart failure.Cardiovasc Res. 2019 Mar 1;115(3):488-500. doi: 10.1093/cvr/cvz010. Cardiovasc Res. 2019. PMID: 30657875 Free PMC article. Review.
-
Potential Strategies for Clinical Translation of Repeated Cell Therapy.Circ Res. 2019 Mar;124(5):690-692. doi: 10.1161/CIRCRESAHA.118.314653. Circ Res. 2019. PMID: 30817255 Free PMC article.
-
Reverse-remodeling after coronary artery bypass grafting in ischemic cardiomyopathy: assessment of myocardial viability by delayed-enhanced magnetic resonance imaging can help cardiac surgeons.Interact Cardiovasc Thorac Surg. 2007 Oct;6(5):673-5. doi: 10.1510/icvts.2007.155275. Epub 2007 Jul 26. Interact Cardiovasc Thorac Surg. 2007. PMID: 17670732
Cited by
-
Real-Time Force and Frequency Analysis of Engineered Human Heart Tissue Derived from Induced Pluripotent Stem Cells Using Magnetic Sensing.Tissue Eng Part C Methods. 2016 Oct;22(10):932-940. doi: 10.1089/ten.TEC.2016.0257. Epub 2016 Sep 28. Tissue Eng Part C Methods. 2016. PMID: 27600722 Free PMC article.
-
Stem cell therapy for CHD: towards translation.Cardiol Young. 2015 Aug;25 Suppl 2(0 2):58-66. doi: 10.1017/S1047951115000840. Cardiol Young. 2015. PMID: 26377711 Free PMC article. Review.
-
New insights into cell-based therapy for heart failure from the CHART-1 study.Eur J Heart Fail. 2017 Nov;19(11):1530-1533. doi: 10.1002/ejhf.955. Epub 2017 Sep 25. Eur J Heart Fail. 2017. PMID: 28948676 Free PMC article. No abstract available.
-
Rebuilding the Damaged Heart: Mesenchymal Stem Cells, Cell-Based Therapy, and Engineered Heart Tissue.Physiol Rev. 2016 Jul;96(3):1127-68. doi: 10.1152/physrev.00019.2015. Physiol Rev. 2016. PMID: 27335447 Free PMC article. Review.
-
Clinical research skills development program in cell-based regenerative medicine.Stem Cells Transl Med. 2015 Feb;4(2):118-22. doi: 10.5966/sctm.2014-0144. Epub 2014 Dec 29. Stem Cells Transl Med. 2015. PMID: 25548389 Free PMC article.
References
-
- Aharinejad S, Abraham D, Paulus P, Zins K, Hofmann M, Michlits W, Gyongyosi M, Macfelda K, Lucas T, Trescher K, Grimm M, Stanley ER. Colony-stimulating factor-1 transfection of myoblasts improves the repair of failing myocardium following autologous myoblast transplantation. Cardiovasc Res 79: 395–404, 2008 - PMC - PubMed
-
- Ahn WS, Jeon JJ, Jeong YR, Lee SJ, Yoon SK. Effect of culture temperature on erythropoietin production and glycosylation in a perfusion culture of recombinant CHO cells. Biotechnol Bioeng 101: 1234–1244, 2008 - PubMed
-
- Amado LC, Saliaris AP, Schuleri KH, John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA 102: 11474–11479, 2005 - PMC - PubMed
-
- Arita NA, Pelaez D, Cheung HS. Activation of the extracellular signal-regulated kinases 1 and 2 (ERK1/2) is needed for the TGFbeta-induced chondrogenic and osteogenic differentiation of mesenchymal stem cells. Biochem Biophys Res Commun 405: 564–569, 2011 - PubMed
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967, 1997 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

