TLR2 signaling subpathways regulate TLR9 signaling for the effective induction of IL-12 upon stimulation by heat-killed Brucella abortus
- PMID: 22635254
- PMCID: PMC4012865
- DOI: 10.1038/cmi.2012.11
TLR2 signaling subpathways regulate TLR9 signaling for the effective induction of IL-12 upon stimulation by heat-killed Brucella abortus
Abstract
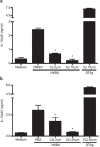
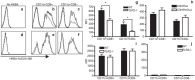
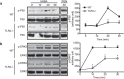
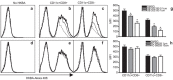
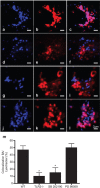
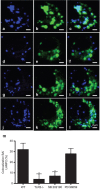
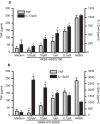
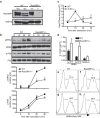
Brucella abortus is a Gram-negative intracellular bacterium that induces MyD88-dependent IL-12 production in dentritic cells (DCs) and a subsequent protective Th1 immune response. Previous studies have shown that the Toll-like receptor 2 (TLR2) is required for tumor-necrosis factor (TNF) production, whereas TLR9 is responsible for IL-12 induction in DCs after exposure to heat-killed Brucella abortus (HKBA). TLR2 is located on the cell surface and is required for optimal microorganism-induced phagocytosis by innate immune cells; thus, phagocytosis is an indispensable preliminary step for bacterial genomic DNA recognition by TLR9 in late-endosomal compartments. Here, we hypothesized that TLR2-triggered signals after HKBA stimulation might cross-regulate TLR9 signaling through the indirect modulation of the phagocytic function of DCs or the direct modulation of cytokine gene expression. Our results indicate that HKBA phagocytosis was TLR2-dependent and an essential step for IL-12p40 induction. In addition, HKBA exposure triggered the TLR2-mediated activation of both p38 and extracellular signal-regulated kinase 1/2 (ERK1/2). Interestingly, although p38 was required for HKBA phagocytosis and phagosome maturation, ERK1/2 did not affect these processes but negatively regulated IL-12 production. Although p38 inhibitors tempered both TNF and IL-12 responses to HKBA, pre-treatment with an ERK1/2 inhibitor significantly increased IL-12p40 and abrogated TNF production in HKBA-stimulated DCs. Further experiments showed that the signaling events that mediated ERK1/2 activation after TLR2 triggering also required HKBA-induced Ras activation. Furthermore, Ras-guanine nucleotide-releasing protein 1 (RasGRP1) mediated the TLR2-induced ERK1/2 activation and inhibition of IL-12p40 production. Taken together, our results demonstrated that HKBA-mediated TLR2-triggering activates both the p38 and ERK1/2 signaling subpathways, which divergently regulate TLR9 activation at several levels to induce an appropriate protective IL-12 response.
Figures
Similar articles
-
The role of innate immune signals in immunity to Brucella abortus.Front Cell Infect Microbiol. 2012 Oct 25;2:130. doi: 10.3389/fcimb.2012.00130. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 23112959 Free PMC article. Review.
-
Heat-killed Brucella abortus induces TNF and IL-12p40 by distinct MyD88-dependent pathways: TNF, unlike IL-12p40 secretion, is Toll-like receptor 2 dependent.J Immunol. 2003 Aug 1;171(3):1441-6. doi: 10.4049/jimmunol.171.3.1441. J Immunol. 2003. PMID: 12874236
-
Th1-like cytokine induction by heat-killed Brucella abortus is dependent on triggering of TLR9.J Immunol. 2005 Sep 15;175(6):3964-70. doi: 10.4049/jimmunol.175.6.3964. J Immunol. 2005. PMID: 16148144
-
TLR2 and TLR4 signaling pathways are required for recombinant Brucella abortus BCSP31-induced cytokine production, functional upregulation of mouse macrophages, and the Th1 immune response in vivo and in vitro.Cell Mol Immunol. 2014 Sep;11(5):477-94. doi: 10.1038/cmi.2014.28. Epub 2014 Apr 28. Cell Mol Immunol. 2014. PMID: 24769793 Free PMC article.
-
Does helminth activation of toll-like receptors modulate immune response in multiple sclerosis patients?Front Cell Infect Microbiol. 2012 Aug 24;2:112. doi: 10.3389/fcimb.2012.00112. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 22937527 Free PMC article. Review.
Cited by
-
Toll-like receptor 6 plays an important role in host innate resistance to Brucella abortus infection in mice.Infect Immun. 2013 May;81(5):1654-62. doi: 10.1128/IAI.01356-12. Epub 2013 Mar 4. Infect Immun. 2013. PMID: 23460520 Free PMC article.
-
The role of innate immune signals in immunity to Brucella abortus.Front Cell Infect Microbiol. 2012 Oct 25;2:130. doi: 10.3389/fcimb.2012.00130. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 23112959 Free PMC article. Review.
-
Ephedrine hydrochloride inhibits PGN-induced inflammatory responses by promoting IL-10 production and decreasing proinflammatory cytokine secretion via the PI3K/Akt/GSK3β pathway.Cell Mol Immunol. 2013 Jul;10(4):330-7. doi: 10.1038/cmi.2013.3. Epub 2013 Apr 22. Cell Mol Immunol. 2013. PMID: 23604046 Free PMC article.
-
Identification of common molecular signatures of SARS-CoV-2 infection and its influence on acute kidney injury and chronic kidney disease.Front Immunol. 2023 Mar 21;14:961642. doi: 10.3389/fimmu.2023.961642. eCollection 2023. Front Immunol. 2023. PMID: 37026010 Free PMC article.
-
Brucella Phagocytosis Mediated by Pathogen-Host Interactions and Their Intracellular Survival.Microorganisms. 2022 Oct 11;10(10):2003. doi: 10.3390/microorganisms10102003. Microorganisms. 2022. PMID: 36296279 Free PMC article. Review.
References
-
- Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. - PubMed
-
- Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. - PubMed
-
- Kawasaki T, Sata T. Perioperative innate immunity and its modulation. J UOEH. 2011;33:123–137. - PubMed
-
- Huang LY, Ishii KJ, Akira S, Aliberti J, Golding B. Th1-like cytokine induction by heat-killed Brucella abortus is dependent on triggering of TLR9. J Immunol. 2005;175:3964–3970. - PubMed
-
- Huang LY, et al. Heat-killed Brucella abortus induces TNF and IL-12p40 by distinct MyD88-dependent pathways: TNF, unlike IL-12p40 secretion, is Toll-like receptor 2 dependent. J Immunol. 2003;171:1441–1446. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

