Initial assessment of the role of CXC chemokine receptor 4 after polytrauma
- PMID: 22634721
- PMCID: PMC3474431
- DOI: 10.2119/molmed.2011.00497
Initial assessment of the role of CXC chemokine receptor 4 after polytrauma
Abstract
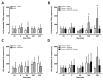
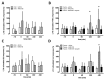
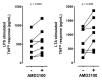
CXC chemokine receptor (CXCR)-4 agonists have been shown to attenuate inflammation and organ injury in various disease models, including trauma/hemorrhage. The pathophysiological role of CXCR4 during the early response to tissue injury, however, remains unknown. Therefore, we investigated the effects of AMD3100, a drug that antagonizes binding of stromal cell-derived factor (SDF)-1α and ubiquitin to CXCR4 during the initial response to polytrauma in pigs. Fifteen minutes before polytrauma (femur fractures/lung contusion; control: sham), 350 nmol/kg AMD3100, equimolar AMD3100 and ubiquitin (350 nmol/kg each) or vehicle were administered intravenously. After a 60-min shock period, fluid resuscitation was performed for 360 min. Ubiquitin binding to peripheral blood mononuclear cells was significantly reduced after intravenous AMD3100. SDF-1α plasma levels increased transiently >10-fold with AMD3100 in all animals. In injured animals, AMD3100 increased fluid requirements to maintain hemodynamics and enhanced increases in peripheral blood granulocytes, lymphocytes and monocytes, compared with its effects in uninjured animals. Cytokine release from leukocytes in response to Toll-like receptor (TLR)-2 and TLR-4 activation was increased after in vitro AMD3100 treatment of normal whole blood and after in vivo AMD3100 administration in animals subjected to polytrauma. Coadministration of AMD3100/ubiquitin reduced lactate levels, prevented AMD3100-induced increases in fluid requirements and sensitization of the tumor necrosis factor (TNF)-α and interleukin (IL)-6 release upon TLR-2/4 activation, but did not attenuate increases in leukocyte counts and SDF-1α plasma levels. Our findings suggest that CXCR4 controls leukocyte mobilization after trauma, regulates leukocyte reactivity toward inflammatory stimuli and mediates protective effects during the early phase of trauma-induced inflammation.
Figures
Similar articles
-
Pharmacological targeting of chemokine (C-X-C motif) receptor 4 in porcine polytrauma and hemorrhage models.J Trauma Acute Care Surg. 2016 Jan;80(1):102-10. doi: 10.1097/TA.0000000000000865. J Trauma Acute Care Surg. 2016. PMID: 26683396 Free PMC article.
-
Effects of exogenous ubiquitin in a polytrauma model with blunt chest trauma.Crit Care Med. 2012 Aug;40(8):2376-84. doi: 10.1097/CCM.0b013e3182514ed9. Crit Care Med. 2012. PMID: 22622399 Free PMC article.
-
MicroRNA-381 Favors Repair of Nerve Injury Through Regulation of the SDF-1/CXCR4 Signaling Pathway via LRRC4 in Acute Cerebral Ischemia after Cerebral Lymphatic Blockage.Cell Physiol Biochem. 2018;46(3):890-906. doi: 10.1159/000488821. Epub 2018 Apr 13. Cell Physiol Biochem. 2018. Retraction in: Cell Physiol Biochem. 2020;54(4):804. doi: 10.33594/000000266 PMID: 29669322 Retracted.
-
Role of CXCR4 chemokine receptor blockade using AMD3100 for mobilization of autologous hematopoietic progenitor cells.Acta Haematol. 2005;114(4):198-205. doi: 10.1159/000088410. Acta Haematol. 2005. PMID: 16269859 Review.
-
Update on clinical experience with AMD3100, an SDF-1/CXCL12-CXCR4 inhibitor, in mobilization of hematopoietic stem and progenitor cells.Curr Opin Hematol. 2010 Jul;17(4):319-26. doi: 10.1097/MOH.0b013e328338b7d5. Curr Opin Hematol. 2010. PMID: 20473162 Review.
Cited by
-
Targeting strategies for delivery of anti-HIV drugs.J Control Release. 2014 Oct 28;192:271-83. doi: 10.1016/j.jconrel.2014.08.003. Epub 2014 Aug 10. J Control Release. 2014. PMID: 25119469 Free PMC article. Review.
-
Pharmacological modulation of C-X-C motif chemokine receptor 4 influences development of acute respiratory distress syndrome after lung ischaemia-reperfusion injury.Clin Exp Pharmacol Physiol. 2018 Jan;45(1):16-26. doi: 10.1111/1440-1681.12845. Epub 2017 Sep 20. Clin Exp Pharmacol Physiol. 2018. PMID: 28815665 Free PMC article.
-
Novel ubiquitin-derived high affinity binding proteins with tumor targeting properties.J Biol Chem. 2014 Mar 21;289(12):8493-507. doi: 10.1074/jbc.M113.519884. Epub 2014 Jan 28. J Biol Chem. 2014. PMID: 24474690 Free PMC article.
-
Pharmacological targeting of chemokine (C-X-C motif) receptor 4 in porcine polytrauma and hemorrhage models.J Trauma Acute Care Surg. 2016 Jan;80(1):102-10. doi: 10.1097/TA.0000000000000865. J Trauma Acute Care Surg. 2016. PMID: 26683396 Free PMC article.
-
Heteromerization of chemokine (C-X-C motif) receptor 4 with α1A/B-adrenergic receptors controls α1-adrenergic receptor function.Proc Natl Acad Sci U S A. 2015 Mar 31;112(13):E1659-68. doi: 10.1073/pnas.1417564112. Epub 2015 Mar 16. Proc Natl Acad Sci U S A. 2015. PMID: 25775528 Free PMC article.
References
-
- Nagasawa T, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–8. - PubMed
-
- Tachibana K, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–4. - PubMed
-
- Karin N. The multiple faces of CXCL12 (SDF-1alpha) in the regulation of immunity during health and disease. J Leukoc Biol. 2010;88:463–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

