Severe hyperhomocysteinemia promotes bone marrow-derived and resident inflammatory monocyte differentiation and atherosclerosis in LDLr/CBS-deficient mice
- PMID: 22628578
- PMCID: PMC3412115
- DOI: 10.1161/CIRCRESAHA.112.269472
Severe hyperhomocysteinemia promotes bone marrow-derived and resident inflammatory monocyte differentiation and atherosclerosis in LDLr/CBS-deficient mice
Abstract
Rationale: Hyperhomocysteinemia (HHcy) accelerates atherosclerosis and increases inflammatory monocytes (MC) in peripheral tissues. However, its causative role in atherosclerosis is not well established and its effect on vascular inflammation has not been studied. The underlying mechanism is unknown.
Objective: This study examined the causative role of HHcy in atherogenesis and its effect on inflammatory MC differentiation.
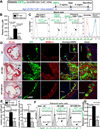
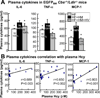
Methods and results: We generated a novel HHcy and hyperlipidemia mouse model, in which cystathionine β-synthase (CBS) and low-density lipoprotein receptor (LDLr) genes were deficient (Ldlr(-/-) Cbs(-/+)). Severe HHcy (plasma homocysteine (Hcy)=275 μmol/L) was induced by a high methionine diet containing sufficient basal levels of B vitamins. Plasma Hcy levels were lowered to 46 μmol/L from 244 μmol/L by vitamin supplementation, which elevated plasma folate levels. Bone marrow (BM)-derived cells were traced by the transplantation of BM cells from enhanced green fluorescent protein (EGFP) transgenic mice after sublethal irradiation of the recipient. HHcy accelerated atherosclerosis and promoted Ly6C(high) inflammatory MC differentiation of both BM and tissue origins in the aortas and peripheral tissues. It also elevated plasma levels of TNF-α, IL-6, and MCP-1; increased vessel wall MC accumulation; and increased macrophage maturation. Hcy-lowering therapy reversed HHcy-induced lesion formation, plasma cytokine increase, and blood and vessel inflammatory MC (Ly6C(high+middle)) accumulation. Plasma Hcy levels were positively correlated with plasma levels of proinflammatory cytokines. In primary mouse splenocytes, L-Hcy promoted rIFNγ-induced inflammatory MC differentiation, as well as increased TNF-α, IL-6, and superoxide anion production in inflammatory MC subsets. Antioxidants and folic acid reversed L-Hcy-induced inflammatory MC differentiation and oxidative stress in inflammatory MC subsets.
Conclusions: HHcy causes vessel wall inflammatory MC differentiation and macrophage maturation of both BM and tissue origins, leading to atherosclerosis via an oxidative stress-related mechanism.
Conflict of interest statement
Figures





Similar articles
-
Hyperhomocysteinemia promotes inflammatory monocyte generation and accelerates atherosclerosis in transgenic cystathionine beta-synthase-deficient mice.Circulation. 2009 Nov 10;120(19):1893-902. doi: 10.1161/CIRCULATIONAHA.109.866889. Epub 2009 Oct 26. Circulation. 2009. PMID: 19858416 Free PMC article.
-
Hyperhomocysteinemia potentiates hyperglycemia-induced inflammatory monocyte differentiation and atherosclerosis.Diabetes. 2014 Dec;63(12):4275-90. doi: 10.2337/db14-0809. Epub 2014 Jul 9. Diabetes. 2014. PMID: 25008174 Free PMC article.
-
Attenuation of early atherogenesis in low-density lipoprotein receptor-deficient mice by proteasome inhibition.Arterioscler Thromb Vasc Biol. 2012 Jun;32(6):1418-26. doi: 10.1161/ATVBAHA.112.249342. Epub 2012 Apr 19. Arterioscler Thromb Vasc Biol. 2012. PMID: 22516063
-
Atherogenesis: hyperhomocysteinemia interactions with LDL, macrophage function, paraoxonase 1, and exercise.Ann N Y Acad Sci. 2016 Jan;1363(1):138-54. doi: 10.1111/nyas.13009. Epub 2016 Feb 5. Ann N Y Acad Sci. 2016. PMID: 26849408 Free PMC article. Review.
-
Hyperhomocysteinemia and atherosclerosis.Sheng Li Xue Bao. 2005 Apr 25;57(2):103-14. Sheng Li Xue Bao. 2005. PMID: 15830093 Review.
Cited by
-
Approaching Inflammation Paradoxes-Proinflammatory Cytokine Blockages Induce Inflammatory Regulators.Front Immunol. 2020 Oct 19;11:554301. doi: 10.3389/fimmu.2020.554301. eCollection 2020. Front Immunol. 2020. PMID: 33193322 Free PMC article.
-
Uremic toxins are conditional danger- or homeostasis-associated molecular patterns.Front Biosci (Landmark Ed). 2018 Jan 1;23(2):348-387. doi: 10.2741/4595. Front Biosci (Landmark Ed). 2018. PMID: 28930551 Free PMC article.
-
Innate immunity of vascular smooth muscle cells contributes to two-wave inflammation in atherosclerosis, twin-peak inflammation in aortic aneurysms and trans-differentiation potential into 25 cell types.Front Immunol. 2024 Jan 24;14:1348238. doi: 10.3389/fimmu.2023.1348238. eCollection 2023. Front Immunol. 2024. PMID: 38327764 Free PMC article.
-
Adaptive Immune Response Signaling Is Suppressed in Ly6Chigh Monocyte but Upregulated in Monocyte Subsets of ApoE-/- Mice - Functional Implication in Atherosclerosis.Front Immunol. 2021 Dec 20;12:809208. doi: 10.3389/fimmu.2021.809208. eCollection 2021. Front Immunol. 2021. PMID: 34987524 Free PMC article.
-
GSNOR modulates hyperhomocysteinemia-induced T cell activation and atherosclerosis by switching Akt S-nitrosylation to phosphorylation.Redox Biol. 2018 Jul;17:386-399. doi: 10.1016/j.redox.2018.04.021. Epub 2018 May 1. Redox Biol. 2018. PMID: 29860106 Free PMC article.
References
-
- Wang H, Jiang X, Yang F, Gaubatz JW, Ma L, Magera MJ, Yang X, Berger PB, Durante W, Pownall HJ, Schafer AI. Hyperhomocysteinemia accelerates atherosclerosis in cystathionine beta-synthase and apolipoprotein E double knock-out mice with and without dietary perturbation. Blood. 2003;101(10):3901–3907. - PubMed
-
- Liao D, Tan H, Hui R, Li Z, Jiang X, Gaubatz J, Yang F, Durante W, Chan L, Schafer AI, Pownall HJ, Yang X, Wang H. Hyperhomocysteinemia decreases circulating high-density lipoprotein by inhibiting apolipoprotein A-I Protein synthesis and enhancing HDL cholesterol clearance. Circ Res. 2006;99(6):598–606. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL82774/HL/NHLBI NIH HHS/United States
- R01 HL108910/HL/NHLBI NIH HHS/United States
- R01 HL077288/HL/NHLBI NIH HHS/United States
- HL108910/HL/NHLBI NIH HHS/United States
- HL77288/HL/NHLBI NIH HHS/United States
- HL67033/HL/NHLBI NIH HHS/United States
- R01 HL082774/HL/NHLBI NIH HHS/United States
- HL57299/HL/NHLBI NIH HHS/United States
- HL11076/HL/NHLBI NIH HHS/United States
- R01 HL094451/HL/NHLBI NIH HHS/United States
- HL94451/HL/NHLBI NIH HHS/United States
- R01 HL057299/HL/NHLBI NIH HHS/United States
- R01 HL067033/HL/NHLBI NIH HHS/United States
- R01 HL110764/HL/NHLBI NIH HHS/United States
- R29 HL057299/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

