Safety and immunogenicity of 2009 pandemic H1N1 influenza vaccination in perinatally HIV-1-infected children, adolescents, and young adults
- PMID: 22615311
- PMCID: PMC3490699
- DOI: 10.1093/infdis/jis360
Safety and immunogenicity of 2009 pandemic H1N1 influenza vaccination in perinatally HIV-1-infected children, adolescents, and young adults
Erratum in
- J Infect Dis. 2012 Dec 15;206(12):1951
Abstract
Background: The safety and immunogenicity of high-dose pandemic H1N1 (pH1N1) vaccination in perinatally human immunodeficiency virus type 1 (HIV-1)-infected children, adolescents, and young adults are unknown.
Methods: Two 30-μg doses of 2009 Novartis pH1N1 monovalent vaccine (Fluvirin) were administered 21-28 days apart to perinatally HIV-1-infected children, adolescents, and young adults. Antibodies were measured by hemagglutination inhibition (HAI) assay at baseline, 21-28 days after first vaccination, 7-13 days after the second vaccination, and 7 months after the first vaccination.
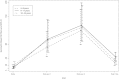
Results: Among the 155 participants, 54 were aged 4-8 years, 51 were aged 9-17 years, and 50 were aged 18-24 years. After 2 doses of Fluvirin, seroresponse (≥ 4-fold rise in HAI titers) was demonstrated in 79.6%, 84.8%, and 83% of participants in the aforementioned age groups, respectively, and seroprotection (HAI titers ≥ 40) was shown in 79.6%, 82.6%, and 85.1%, respectively. Of those lacking seroresponse (n = 43) or seroprotection (n = 37) after the first vaccination, 46.5% and 40.5% achieved seroresponse or seroprotection, respectively, after the second vaccination. Among participants who lacked seroprotection at entry, a "complete response" (both seroresponse and seroprotection) after first vaccination was associated with higher baseline log(10) HAI titer and non-Hispanic ethnicity. No serious vaccine-related events occurred.
Conclusion: Two doses of double-strength pH1N1 vaccine are safe and immunogenic and may provide improved protection against influenza in perinatally HIV-1-infected children and youth.
Clinical trials registration: NCT00992836.
Figures
Similar articles
-
Safety and immunogenicity of 2009 pH1N1 vaccination in HIV-infected pregnant women.Clin Infect Dis. 2013 May;56(10):1488-97. doi: 10.1093/cid/cit057. Epub 2013 Feb 1. Clin Infect Dis. 2013. PMID: 23378284 Free PMC article. Clinical Trial.
-
Immunogenicity and safety of monovalent influenza A (H1N1) 2009 in HIV-infected Thai children.Vaccine. 2011 Nov 3;29(47):8705-11. doi: 10.1016/j.vaccine.2011.08.101. Epub 2011 Sep 3. Vaccine. 2011. PMID: 21893147
-
Safety and immunogenicity of a monovalent MF59®-adjuvanted A/H1N1 vaccine in HIV-infected children and young adults.Biologicals. 2012 Mar;40(2):134-9. doi: 10.1016/j.biologicals.2011.12.001. Epub 2012 Jan 20. Biologicals. 2012. PMID: 22261282 Clinical Trial.
-
Response to 2009 pandemic influenza A (H1N1) vaccine in HIV-infected patients and the influence of prior seasonal influenza vaccination.PLoS One. 2011 Jan 31;6(1):e16496. doi: 10.1371/journal.pone.0016496. PLoS One. 2011. PMID: 21304982 Free PMC article. Clinical Trial.
-
Long-term immune responses to vaccination in HIV-infected patients: a systematic review and meta-analysis.Clin Infect Dis. 2014 Apr;58(8):1130-9. doi: 10.1093/cid/cit937. Epub 2014 Jan 10. Clin Infect Dis. 2014. PMID: 24415637 Free PMC article. Review.
Cited by
-
Transcriptional and Immunologic Correlates of Response to Pandemic Influenza Vaccine in Aviremic, HIV-Infected Children.Front Immunol. 2021 Mar 25;12:639358. doi: 10.3389/fimmu.2021.639358. eCollection 2021. Front Immunol. 2021. PMID: 33868267 Free PMC article.
-
Safety and immunogenicity of 2009 pH1N1 vaccination in HIV-infected pregnant women.Clin Infect Dis. 2013 May;56(10):1488-97. doi: 10.1093/cid/cit057. Epub 2013 Feb 1. Clin Infect Dis. 2013. PMID: 23378284 Free PMC article. Clinical Trial.
-
Influenza A Virus Infection and Nucleotide Sequencing in HIV-Infected Children: A Case Report and Review of Literature.Glob Pediatr Health. 2017 Jul 12;4:2333794X17719203. doi: 10.1177/2333794X17719203. eCollection 2017. Glob Pediatr Health. 2017. PMID: 28812054 Free PMC article. No abstract available.
-
Effect of abatacept on immunogenicity of vaccines in individuals with type 1 diabetes.Vaccine. 2013 Oct 1;31(42):4791-4. doi: 10.1016/j.vaccine.2013.07.086. Epub 2013 Aug 17. Vaccine. 2013. PMID: 23962535 Free PMC article. Clinical Trial.
-
Immune Responses to Circulating and Vaccine Viral Strains in HIV-Infected and Uninfected Children and Youth Who Received the 2013/2014 Quadrivalent Live-Attenuated Influenza Vaccine.Front Immunol. 2016 Apr 15;7:142. doi: 10.3389/fimmu.2016.00142. eCollection 2016. Front Immunol. 2016. PMID: 27148262 Free PMC article.
References
-
- Dawood FS, Jain S, Finelli L, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15. - PubMed
-
- Gatherer D. The 2009 H1N1 influenza outbreak in its historical context. J Clin Virol. 2009;45:174–8. - PubMed
-
- World Health Organization. Global Alert and Response: Situation Updates (Pandemic) H1N1 2009. http://www.who.int/csr/disease/swineflu/updates/en/index.html. Accessed May 29, 2012.
-
- Bautista E, Chotpitayasunondh T, Gao Z, et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362:1708–19. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- HHSN267200800001G/DK/NIDDK NIH HHS/United States
- U01 AI068632/AI/NIAID NIH HHS/United States
- UL1 RR025771/RR/NCRR NIH HHS/United States
- U01 AI068616/AI/NIAID NIH HHS/United States
- HHSN267200800001C/HD/NICHD NIH HHS/United States
- U01 AI041110/AI/NIAID NIH HHS/United States
- N01-DK-9-001/HHSN267200800001C/DK/NIDDK NIH HHS/United States
- AI068632/AI/NIAID NIH HHS/United States
- UM1 AI069536/AI/NIAID NIH HHS/United States
- UL1 RR029893/RR/NCRR NIH HHS/United States
- UM1 AI068632/AI/NIAID NIH HHS/United States
- 1 U01 AI068616/AI/NIAID NIH HHS/United States
- 5 U01 AI41110/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical