PINK1 as a molecular checkpoint in the maintenance of mitochondrial function and integrity
- PMID: 22610403
- PMCID: PMC3887784
- DOI: 10.1007/s10059-012-0100-8
PINK1 as a molecular checkpoint in the maintenance of mitochondrial function and integrity
Abstract
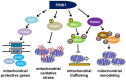
Parkinson's disease (PD), the most prevalent neurodegenerative movement disorder, is characterized by an age-dependent selective loss of dopaminergic (DA) neurons. Although most PD cases are sporadic, more than 20 responsible genes in familial cases were identified recently. Genetic studies using Drosophila models demonstrate that PINK1, a mitochondrial kinase encoded by a PD-linked gene PINK1, is critical for maintaining mitochondrial function and integrity. This suggests that mitochondrial dysfunction is the main cause of PD pathogenesis. Further genetic and cell biological studies revealed that PINK1 recruits Parkin, an E3 ubiquitin ligase encoded by another PD-linked gene parkin, to mitochondria and regulates the mitochondrial remodeling process via the Parkin-mediated ubiquitination of various mitochondrial proteins. PINK1 also directly phosphorylates the mitochondrial proteins Miro and TRAP1, subsequently inhibiting mitochondrial transport and mitochondrial oxidative damage, respectively. Moreover, recent Drosophila genetic analyses demonstrate that the neuroprotective molecules Sir2 and FOXO specifically complement mitochondrial dysfunction and DA neuron loss in PINK1 null mutants, suggesting that Sir2 and FOXO protect mitochondria and DA neurons downstream of PINK1. Collectively, these recent results suggest that PINK1 plays multiple roles in mitochondrial quality control by regulating its mitochondrial, cytosolic, and nuclear targets.
Figures
Similar articles
-
Silent information regulator 2 (Sir2) and Forkhead box O (FOXO) complement mitochondrial dysfunction and dopaminergic neuron loss in Drosophila PTEN-induced kinase 1 (PINK1) null mutant.J Biol Chem. 2012 Apr 13;287(16):12750-8. doi: 10.1074/jbc.M111.337907. Epub 2012 Feb 29. J Biol Chem. 2012. PMID: 22378780 Free PMC article.
-
Parkinson's disease-associated kinase PINK1 regulates Miro protein level and axonal transport of mitochondria.PLoS Genet. 2012;8(3):e1002537. doi: 10.1371/journal.pgen.1002537. Epub 2012 Mar 1. PLoS Genet. 2012. PMID: 22396657 Free PMC article.
-
Drosophila Trap1 protects against mitochondrial dysfunction in a PINK1/parkin model of Parkinson's disease.Cell Death Dis. 2013 Jan 17;4(1):e467. doi: 10.1038/cddis.2012.205. Cell Death Dis. 2013. PMID: 23328674 Free PMC article.
-
PINK1-Parkin signaling in Parkinson's disease: Lessons from Drosophila.Neurosci Res. 2020 Oct;159:40-46. doi: 10.1016/j.neures.2020.01.016. Epub 2020 Feb 6. Neurosci Res. 2020. PMID: 32035987 Review.
-
Impaired mitochondrial dynamics and function in the pathogenesis of Parkinson's disease.Exp Neurol. 2009 Aug;218(2):235-46. doi: 10.1016/j.expneurol.2009.03.006. Epub 2009 Mar 18. Exp Neurol. 2009. PMID: 19303005 Review.
Cited by
-
Emerging functional cross-talk between the Keap1-Nrf2 system and mitochondria.J Clin Biochem Nutr. 2015 Mar;56(2):91-7. doi: 10.3164/jcbn.14-134. Epub 2015 Mar 1. J Clin Biochem Nutr. 2015. PMID: 25759513 Free PMC article. Review.
-
The Mitochondrial Hsp90 TRAP1 and Alzheimer's Disease.Front Mol Biosci. 2021 Jun 18;8:697913. doi: 10.3389/fmolb.2021.697913. eCollection 2021. Front Mol Biosci. 2021. PMID: 34222342 Free PMC article. Review.
-
Characterization of PINK1 (PTEN-induced putative kinase 1) mutations associated with Parkinson disease in mammalian cells and Drosophila.J Biol Chem. 2013 Feb 22;288(8):5660-72. doi: 10.1074/jbc.M112.430801. Epub 2013 Jan 9. J Biol Chem. 2013. PMID: 23303188 Free PMC article.
-
Aconitase causes iron toxicity in Drosophila pink1 mutants.PLoS Genet. 2013 Apr;9(4):e1003478. doi: 10.1371/journal.pgen.1003478. Epub 2013 Apr 25. PLoS Genet. 2013. PMID: 23637640 Free PMC article.
-
Genetic and pharmacologic p32-inhibition rescue CHCHD2-linked Parkinson's disease phenotypes in vivo and in cell models.J Biomed Sci. 2024 Feb 23;31(1):24. doi: 10.1186/s12929-024-01010-z. J Biomed Sci. 2024. PMID: 38395904 Free PMC article.
References
-
- Araki T., Sasaki Y., Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. - PubMed
-
- Betarbet R., Sherer T.B., MacKenzie G., Garcia-Osuna M., Panov A.V., Greenamyre J.T. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000;3:1301–1306. - PubMed
-
- Bonifati V., Rizzu P., van Baren M.J., Schaap O., Breedveld G.J., Krieger E., Dekker M.C.J., Squitieri F., Ibanez P., Joosse M., et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. - PubMed
-
- Brooks A.I., Chadwick C.A., Gelbard H.A., Cory-Slechta D.A., Federoff H.J. Paraquat elicited neurobehavioral syndrome caused by dopaminergic neuron loss. Brain Res. 1999;823:1–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

