FMNL2 drives actin-based protrusion and migration downstream of Cdc42
- PMID: 22608513
- PMCID: PMC3765947
- DOI: 10.1016/j.cub.2012.03.064
FMNL2 drives actin-based protrusion and migration downstream of Cdc42
Abstract
Cell migration entails protrusion of lamellipodia, densely packed networks of actin filaments at the cell front. Filaments are generated by nucleation, likely mediated by Arp2/3 complex and its activator Scar/WAVE. It is unclear whether formins contribute to lamellipodial actin filament nucleation or serve as elongators of filaments nucleated by Arp2/3 complex. Here we show that the Diaphanous-related formin FMNL2, also known as FRL3 or FHOD2, accumulates at lamellipodia and filopodia tips. FMNL2 is cotranslationally modified by myristoylation and regulated by interaction with the Rho-guanosine triphosphatase Cdc42. Abolition of myristoylation or Cdc42 binding interferes with proper FMNL2 activation, constituting an essential prerequisite for subcellular targeting. In vitro, C-terminal FMNL2 drives elongation rather than nucleation of actin filaments in the presence of profilin. In addition, filament ends generated by Arp2/3-mediated branching are captured and efficiently elongated by the formin. Consistent with these biochemical properties, RNAi-mediated silencing of FMNL2 expression decreases the rate of lamellipodia protrusion and, accordingly, the efficiency of cell migration. Our data establish that the FMNL subfamily member FMNL2 is a novel elongation factor of actin filaments that constitutes the first Cdc42 effector promoting cell migration and actin polymerization at the tips of lamellipodia.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
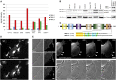
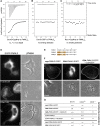
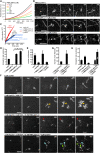

Comment in
-
Actin cytoskeleton: a team effort during actin assembly.Curr Biol. 2012 Aug 21;22(16):R643-5. doi: 10.1016/j.cub.2012.07.026. Curr Biol. 2012. PMID: 22917514
Similar articles
-
FMNL formins boost lamellipodial force generation.Nat Commun. 2017 Mar 22;8:14832. doi: 10.1038/ncomms14832. Nat Commun. 2017. PMID: 28327544 Free PMC article.
-
The structure of FMNL2-Cdc42 yields insights into the mechanism of lamellipodia and filopodia formation.Nat Commun. 2015 May 12;6:7088. doi: 10.1038/ncomms8088. Nat Commun. 2015. PMID: 25963737 Free PMC article.
-
FMNL2 and -3 regulate Golgi architecture and anterograde transport downstream of Cdc42.Sci Rep. 2017 Aug 29;7(1):9791. doi: 10.1038/s41598-017-09952-1. Sci Rep. 2017. PMID: 28852060 Free PMC article.
-
Actin dynamics in cell migration.Essays Biochem. 2019 Oct 31;63(5):483-495. doi: 10.1042/EBC20190015. Essays Biochem. 2019. PMID: 31551324 Free PMC article. Review.
-
Formins as effector proteins of Rho GTPases.Small GTPases. 2014;5:e29513. doi: 10.4161/sgtp.29513. Epub 2014 Jun 10. Small GTPases. 2014. PMID: 24914801 Free PMC article. Review.
Cited by
-
Comparative gene expression analysis of the fmnl family of formins during zebrafish development and implications for tissue specific functions.Gene Expr Patterns. 2013 Jan-Feb;13(1-2):30-7. doi: 10.1016/j.gep.2012.09.002. Epub 2012 Oct 13. Gene Expr Patterns. 2013. PMID: 23072729 Free PMC article.
-
Protein-N-myristoylation-dependent phosphorylation of serine 13 of tyrosine kinase Lyn by casein kinase 1γ at the Golgi during intracellular protein traffic.Sci Rep. 2020 Oct 1;10(1):16273. doi: 10.1038/s41598-020-73248-0. Sci Rep. 2020. PMID: 33004926 Free PMC article.
-
The formins FMNL1 and mDia1 regulate coiling phagocytosis of Borrelia burgdorferi by primary human macrophages.Infect Immun. 2013 May;81(5):1683-95. doi: 10.1128/IAI.01411-12. Epub 2013 Mar 4. Infect Immun. 2013. PMID: 23460512 Free PMC article.
-
Junctional actin assembly is mediated by Formin-like 2 downstream of Rac1.J Cell Biol. 2015 May 11;209(3):367-76. doi: 10.1083/jcb.201412015. J Cell Biol. 2015. PMID: 25963818 Free PMC article.
-
Formin-like2 regulates Rho/ROCK pathway to promote actin assembly and cell invasion of colorectal cancer.Cancer Sci. 2015 Oct;106(10):1385-93. doi: 10.1111/cas.12768. Cancer Sci. 2015. Retraction in: Cancer Sci. 2016 Jul;107(7):1060. doi: 10.1111/cas.12972 PMID: 26258642 Free PMC article. Retracted.
References
-
- Ridley A.J. Life at the leading edge. Cell. 2011;145:1012–1022. - PubMed
-
- Schönichen A., Geyer M. Fifteen formins for an actin filament: a molecular view on the regulation of human formins. Biochim. Biophys. Acta. 2010;1803:152–163. - PubMed
-
- Chesarone M.A., DuPage A.G., Goode B.L. Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat. Rev. Mol. Cell Biol. 2010;11:62–74. - PubMed
-
- Faix J., Rottner K. The making of filopodia. Curr. Opin. Cell Biol. 2006;18:18–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

