Mechanisms controlling the guidance of thalamocortical axons through the embryonic forebrain
- PMID: 22607003
- PMCID: PMC4370206
- DOI: 10.1111/j.1460-9568.2012.08119.x
Mechanisms controlling the guidance of thalamocortical axons through the embryonic forebrain
Abstract
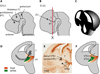
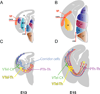
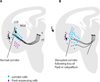
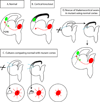
Thalamocortical axons must cross a complex cellular terrain through the developing forebrain, and this terrain has to be understood for us to learn how thalamocortical axons reach their destinations. Selective fasciculation, guidepost cells and various diencephalic and telencephalic gradients have been implicated in thalamocortical guidance. As our understanding of the relevant forebrain patterns has increased, so has our knowledge of the guidance mechanisms. Our aim here is to review recent observations of cellular and molecular mechanisms related to: the growth of thalamofugal projections to the ventral telencephalon, thalamic axon avoidance of the hypothalamus and extension into the telencephalon to form the internal capsule, the crossing of the pallial-subpallial boundary, and the growth towards the cerebral cortex. We shall review current theories for the explanation of the maintenance and alteration of topographic order in the thalamocortical projections to the cortex. It is now increasingly clear that several mechanisms are involved at different stages of thalamocortical development, and each contributes substantially to the eventual outcome. Revealing the molecular and cellular mechanisms can help to link specific genes to details of actual developmental mechanisms.
© 2012 The Authors. European Journal of Neuroscience © 2012 Federation of European Neuroscience Societies and Blackwell Publishing Ltd.
Figures

Similar articles
-
Evidence that descending cortical axons are essential for thalamocortical axons to cross the pallial-subpallial boundary in the embryonic forebrain.PLoS One. 2012;7(3):e33105. doi: 10.1371/journal.pone.0033105. Epub 2012 Mar 8. PLoS One. 2012. PMID: 22412988 Free PMC article.
-
Normal ventral telencephalic expression of Pax6 is required for normal development of thalamocortical axons in embryonic mice.Neural Dev. 2009 Jun 5;4:19. doi: 10.1186/1749-8104-4-19. Neural Dev. 2009. PMID: 19500363 Free PMC article.
-
OL-Protocadherin is essential for growth of striatal axons and thalamocortical projections.Nat Neurosci. 2007 Sep;10(9):1151-9. doi: 10.1038/nn1960. Epub 2007 Aug 26. Nat Neurosci. 2007. PMID: 17721516
-
Development and Evolution of Thalamocortical Connectivity.Cold Spring Harb Perspect Biol. 2024 Jan 2;16(1):a041503. doi: 10.1101/cshperspect.a041503. Cold Spring Harb Perspect Biol. 2024. PMID: 38167425 Review.
-
Inputs from the thalamocortical system on axon pathfinding mechanisms.Curr Opin Neurobiol. 2014 Aug;27:143-50. doi: 10.1016/j.conb.2014.03.013. Epub 2014 Apr 17. Curr Opin Neurobiol. 2014. PMID: 24742382 Review.
Cited by
-
Chromatin remodeler Arid1a regulates subplate neuron identity and wiring of cortical connectivity.Proc Natl Acad Sci U S A. 2021 May 25;118(21):e2100686118. doi: 10.1073/pnas.2100686118. Proc Natl Acad Sci U S A. 2021. PMID: 34011608 Free PMC article.
-
Growth of Thalamocortical Fibers to the Somatosensory Cortex in the Human Fetal Brain.Front Neurosci. 2017 Apr 27;11:233. doi: 10.3389/fnins.2017.00233. eCollection 2017. Front Neurosci. 2017. PMID: 28496398 Free PMC article.
-
Pathfinding of corticothalamic axons relies on a rendezvous with thalamic projections.Neuron. 2013 Feb 6;77(3):472-84. doi: 10.1016/j.neuron.2012.11.031. Neuron. 2013. PMID: 23395374 Free PMC article.
-
Integrative mechanisms of oriented neuronal migration in the developing brain.Annu Rev Cell Dev Biol. 2013;29:299-353. doi: 10.1146/annurev-cellbio-101512-122400. Epub 2013 Aug 7. Annu Rev Cell Dev Biol. 2013. PMID: 23937349 Free PMC article. Review.
-
EphA4 has distinct functionality from EphA7 in the corticothalamic system during mouse brain development.J Comp Neurol. 2016 Jul 1;524(10):2080-92. doi: 10.1002/cne.23933. Epub 2015 Dec 3. J Comp Neurol. 2016. PMID: 26587807 Free PMC article.
References
-
- Adams NC, Baker GE. Cells of the perireticular nucleus project to the developing neocortex of the rat. J Comp Neurol. 1995 Sep 4;359(4):613–626. 1995. - PubMed
-
- Adams NC, Lozsádi DA, Guillery RW. Complexities in the thalamocortical and corticothalamic pathways. Eur J Neurosci. 1997;9(2):204–209. - PubMed
-
- Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu Rev Neurosci. 1994;17:185–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

