Phage displayed peptides/antibodies recognizing growth factors and their tyrosine kinase receptors as tools for anti-cancer therapeutics
- PMID: 22606042
- PMCID: PMC3344278
- DOI: 10.3390/ijms13045254
Phage displayed peptides/antibodies recognizing growth factors and their tyrosine kinase receptors as tools for anti-cancer therapeutics
Abstract
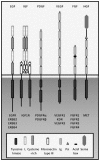
The basic idea of displaying peptides on a phage, introduced by George P. Smith in 1985, was greatly developed and improved by McCafferty and colleagues at the MRC Laboratory of Molecular Biology and, later, by Barbas and colleagues at the Scripps Research Institute. Their approach was dedicated to building a system for the production of antibodies, similar to a naïve B cell repertoire, in order to by-pass the standard hybridoma technology that requires animal immunization. Both groups merged the phage display technology with an antibody library to obtain a huge number of phage variants, each of them carrying a specific antibody ready to bind its target molecule, allowing, later on, rare phage (one in a million) to be isolated by affinity chromatography. Here, we will briefly review the basis of the technology and the therapeutic application of phage-derived bioactive molecules when addressed against key players in tumor development and progression: growth factors and their tyrosine kinase receptors.
Keywords: anticancer therapy; growth factors; humanized antibody; phage display; tyrosine kinase receptors.
Figures
Similar articles
-
A Novel Artificially Humanized Anti-Cripto-1 Antibody Suppressing Cancer Cell Growth.Int J Mol Sci. 2021 Feb 8;22(4):1709. doi: 10.3390/ijms22041709. Int J Mol Sci. 2021. PMID: 33567764 Free PMC article.
-
Generation of Recombinant Antibodies Against Toxins and Viruses by Phage Display for Diagnostics and Therapy.Adv Exp Med Biol. 2016;917:55-76. doi: 10.1007/978-3-319-32805-8_4. Adv Exp Med Biol. 2016. PMID: 27236552 Free PMC article. Review.
-
Phage Display Technology as a Powerful Platform for Antibody Drug Discovery.Viruses. 2021 Jan 25;13(2):178. doi: 10.3390/v13020178. Viruses. 2021. PMID: 33504115 Free PMC article. Review.
-
The selection performance of an antibody library displayed on filamentous phage coat proteins p9, p3 and truncated p3.BMC Res Notes. 2014 Sep 19;7:661. doi: 10.1186/1756-0500-7-661. BMC Res Notes. 2014. PMID: 25238965 Free PMC article.
-
Harnessing Phage Display for the Discovery of Peptide-Based Drugs and Monoclonal Antibodies.Curr Med Chem. 2021;28(40):8267-8274. doi: 10.2174/0929867327666201111144353. Curr Med Chem. 2021. PMID: 33176631 Review.
Cited by
-
Strategies of targeting the extracellular domain of RON tyrosine kinase receptor for cancer therapy and drug delivery.J Cancer Res Clin Oncol. 2016 Dec;142(12):2429-2446. doi: 10.1007/s00432-016-2214-4. Epub 2016 Aug 8. J Cancer Res Clin Oncol. 2016. PMID: 27503093 Review.
-
Phage-Displayed Peptides for Targeting Tyrosine Kinase Membrane Receptors in Cancer Therapy.Viruses. 2021 Apr 9;13(4):649. doi: 10.3390/v13040649. Viruses. 2021. PMID: 33918836 Free PMC article. Review.
-
An Appraisal of Bacteriophage Isolation Techniques from Environment.Microb Ecol. 2022 Apr;83(3):519-535. doi: 10.1007/s00248-021-01782-z. Epub 2021 Jun 17. Microb Ecol. 2022. PMID: 34136953 Review.
References
-
- Edelman G.M. Antibody structure and molecular immunology. Science. 1973;180:830–840. - PubMed
-
- Skerra A., Pluckthun A. Assembly of a functional immunoglobulin Fv fragment in Escherichia coli. Science. 1988;240:1038–1041. - PubMed
-
- McCafferty J., Griffiths A.D., Winter G., Chiswell D.J. Phage antibodies: Filamentous phage displaying antibody variable domains. Nature. 1990;348:552–554. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources