The nonskeletal effects of vitamin D: an Endocrine Society scientific statement
- PMID: 22596255
- PMCID: PMC3365859
- DOI: 10.1210/er.2012-1000
The nonskeletal effects of vitamin D: an Endocrine Society scientific statement
Abstract
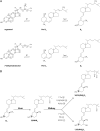
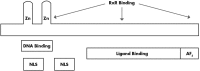
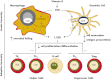
Significant controversy has emerged over the last decade concerning the effects of vitamin D on skeletal and nonskeletal tissues. The demonstration that the vitamin D receptor is expressed in virtually all cells of the body and the growing body of observational data supporting a relationship of serum 25-hydroxyvitamin D to chronic metabolic, cardiovascular, and neoplastic diseases have led to widespread utilization of vitamin D supplementation for the prevention and treatment of numerous disorders. In this paper, we review both the basic and clinical aspects of vitamin D in relation to nonskeletal organ systems. We begin by focusing on the molecular aspects of vitamin D, primarily by examining the structure and function of the vitamin D receptor. This is followed by a systematic review according to tissue type of the inherent biological plausibility, the strength of the observational data, and the levels of evidence that support or refute an association between vitamin D levels or supplementation and maternal/child health as well as various disease states. Although observational studies support a strong case for an association between vitamin D and musculoskeletal, cardiovascular, neoplastic, and metabolic disorders, there remains a paucity of large-scale and long-term randomized clinical trials. Thus, at this time, more studies are needed to definitively conclude that vitamin D can offer preventive and therapeutic benefits across a wide range of physiological states and chronic nonskeletal disorders.
Figures
Similar articles
-
Skeletal and nonskeletal effects of vitamin D: is vitamin D a tonic for bone and other tissues?Osteoporos Int. 2014 Oct;25(10):2347-57. doi: 10.1007/s00198-014-2749-7. Epub 2014 May 21. Osteoporos Int. 2014. PMID: 24846318 Review.
-
Effectiveness and safety of vitamin D in relation to bone health.Evid Rep Technol Assess (Full Rep). 2007 Aug;(158):1-235. Evid Rep Technol Assess (Full Rep). 2007. PMID: 18088161 Free PMC article. Review.
-
Non-classical effects of vitamin D: Non-bone effects of vitamin D.Ann Endocrinol (Paris). 2021 Feb;82(1):43-51. doi: 10.1016/j.ando.2020.12.002. Epub 2020 Dec 3. Ann Endocrinol (Paris). 2021. PMID: 33279474 Review.
-
Vitamin D: an essential component for skeletal health.Ann N Y Acad Sci. 2011 Dec;1240:E1-12. doi: 10.1111/j.1749-6632.2011.06374.x. Ann N Y Acad Sci. 2011. PMID: 22360357
-
A Prospective Study to Evaluate the Possible Role of Cholecalciferol Supplementation on Autoimmunity in Hashimoto's Thyroiditis.J Assoc Physicians India. 2023 Jan;71(1):1. J Assoc Physicians India. 2023. PMID: 37116030 Clinical Trial.
Cited by
-
Vitamin D and cardiovascular disease: is the evidence solid?Eur Heart J. 2013 Dec;34(48):3691-8. doi: 10.1093/eurheartj/eht166. Epub 2013 Jun 9. Eur Heart J. 2013. PMID: 23751422 Free PMC article. Review.
-
Vitamin D: not just the bone. Evidence for beneficial pleiotropic extraskeletal effects.Eat Weight Disord. 2017 Mar;22(1):27-41. doi: 10.1007/s40519-016-0312-6. Epub 2016 Aug 23. Eat Weight Disord. 2017. PMID: 27553017 Review.
-
Circulating Cathelicidin Concentrations in a Cohort of Healthy Children: Influence of Age, Body Composition, Gender and Vitamin D Status.PLoS One. 2016 May 6;11(5):e0152711. doi: 10.1371/journal.pone.0152711. eCollection 2016. PLoS One. 2016. PMID: 27152524 Free PMC article.
-
A Cross-sectional Examination of Vitamin D, Obesity, and Measures of Pain and Function in Middle-aged and Older Adults With Knee Osteoarthritis.Clin J Pain. 2015 Dec;31(12):1060-7. doi: 10.1097/AJP.0000000000000210. Clin J Pain. 2015. PMID: 25569220 Free PMC article.
-
Status of research on vitamin D supplementation in treating or preventing liver fibrosis.Liver Int. 2013 May;33(5):653-5. doi: 10.1111/liv.12147. Liver Int. 2013. PMID: 23560728 Free PMC article. No abstract available.
References
-
- Rosen CJ. 2011. Vitamin D insufficiency. N Engl J Med 364:248–254 - PubMed
-
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. 2011. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 96:1911–1930 - PubMed
-
- IOM (Institute of Medicine) 2011. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press - PubMed
-
- McDonnell DP, Mangelsdorf DJ, Pike JW, Haussler MR, O'Malley BW. 1987. Molecular cloning of complementary DNA encoding the avian receptor for vitamin D. Science 235:1214–1217 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 RR033176/RR/NCRR NIH HHS/United States
- R01 AR050023/AR/NIAMS NIH HHS/United States
- R21 AR056885/AR/NIAMS NIH HHS/United States
- MOP-84253/CAPMC/ CIHR/Canada
- R01AI073539/AI/NIAID NIH HHS/United States
- UL1 TR000124/TR/NCATS NIH HHS/United States
- I01 BX001066/BX/BLRD VA/United States
- CA13896/CA/NCI NIH HHS/United States
- DK092759/DK/NIDDK NIH HHS/United States
- R01 AI073539/AI/NIAID NIH HHS/United States
- UL1RR033176/RR/NCRR NIH HHS/United States
- UL1TR000124/TR/NCATS NIH HHS/United States
- DK46974/DK/NIDDK NIH HHS/United States
- R24 DK092759/DK/NIDDK NIH HHS/United States
- T32 AR059033/AR/NIAMS NIH HHS/United States
- U01 CA138962/CA/NCI NIH HHS/United States
- T32AR059033/AR/NIAMS NIH HHS/United States
- R01 DK046974/DK/NIDDK NIH HHS/United States
- R21AR056885/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

