Calcium-dependent but action potential-independent BCM-like metaplasticity in the hippocampus
- PMID: 22593048
- PMCID: PMC6622209
- DOI: 10.1523/JNEUROSCI.0634-12.2012
Calcium-dependent but action potential-independent BCM-like metaplasticity in the hippocampus
Abstract
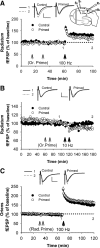
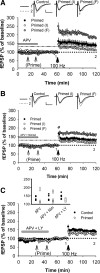
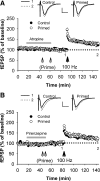
The Bienenstock, Cooper and Munro (BCM) computational model, which incorporates a metaplastic sliding threshold for LTP induction, accounts well for experience-dependent changes in synaptic plasticity in the visual cortex. BCM-like metaplasticity over a shorter timescale has also been observed in the hippocampus, thus providing a tractable experimental preparation for testing specific predictions of the model. Here, using extracellular and intracellular electrophysiological recordings from acute rat hippocampal slices, we tested the critical BCM predictions (1) that high levels of synaptic activation will induce a metaplastic state that spreads across dendritic compartments, and (2) that postsynaptic cell-firing is the critical trigger for inducing that state. In support of the first premise, high-frequency priming stimulation inhibited subsequent long-term potentiation and facilitated subsequent long-term depression at synapses quiescent during priming, including those located in a dendritic compartment different to that of the primed pathway. These effects were not dependent on changes in synaptic inhibition or NMDA/metabotropic glutamate receptor function. However, in contrast to the BCM prediction, somatic action potentials during priming were neither necessary nor sufficient to induce the metaplasticity effect. Instead, in broad agreement with derivatives of the BCM model, calcium as released from intracellular stores and triggered by M1 muscarinic acetylcholine receptor activation was critical for altering subsequent synaptic plasticity. These results indicate that synaptic plasticity in stratum radiatum of CA1 can be homeostatically regulated by the cell-wide history of synaptic activity through a calcium-dependent but action potential-independent mechanism.
Figures




Similar articles
-
Activation of Group II Metabotropic Glutamate Receptors Promotes LTP Induction at Schaffer Collateral-CA1 Pyramidal Cell Synapses by Priming NMDA Receptors.J Neurosci. 2016 Nov 9;36(45):11521-11531. doi: 10.1523/JNEUROSCI.1519-16.2016. J Neurosci. 2016. PMID: 27911756 Free PMC article.
-
Heterosynaptic metaplasticity in the hippocampus in vivo: a BCM-like modifiable threshold for LTP.Proc Natl Acad Sci U S A. 2001 Sep 11;98(19):10924-9. doi: 10.1073/pnas.181342098. Epub 2001 Aug 21. Proc Natl Acad Sci U S A. 2001. PMID: 11517323 Free PMC article.
-
Synaptic strength at the temporoammonic input to the hippocampal CA1 region in vivo is regulated by NMDA receptors, metabotropic glutamate receptors and voltage-gated calcium channels.Neuroscience. 2015 Nov 19;309:191-9. doi: 10.1016/j.neuroscience.2015.03.014. Epub 2015 Mar 17. Neuroscience. 2015. PMID: 25791230
-
Synaptic plasticity, metaplasticity and BCM theory.Bratisl Lek Listy. 2002;103(4-5):137-43. Bratisl Lek Listy. 2002. PMID: 12413200 Review.
-
Metaplasticity: a new vista across the field of synaptic plasticity.Prog Neurobiol. 1997 Jul;52(4):303-23. doi: 10.1016/s0301-0082(97)00018-x. Prog Neurobiol. 1997. PMID: 9247968 Review.
Cited by
-
Astrocytes and synaptic plasticity in health and disease.Exp Brain Res. 2017 Jun;235(6):1645-1655. doi: 10.1007/s00221-017-4928-1. Epub 2017 Mar 15. Exp Brain Res. 2017. PMID: 28299411 Review.
-
TNF-Mediated Homeostatic Synaptic Plasticity: From in vitro to in vivo Models.Front Cell Neurosci. 2020 Sep 30;14:565841. doi: 10.3389/fncel.2020.565841. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33192311 Free PMC article. Review.
-
Bridging Synaptic and Epigenetic Maintenance Mechanisms of the Engram.Front Mol Neurosci. 2018 Oct 5;11:369. doi: 10.3389/fnmol.2018.00369. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30344478 Free PMC article.
-
Pathway-specific TNF-mediated metaplasticity in hippocampal area CA1.Sci Rep. 2022 Feb 2;12(1):1746. doi: 10.1038/s41598-022-05844-1. Sci Rep. 2022. PMID: 35110639 Free PMC article.
-
Metaplasticity framework for cross-modal synaptic plasticity in adults.Front Synaptic Neurosci. 2023 Jan 6;14:1087042. doi: 10.3389/fnsyn.2022.1087042. eCollection 2022. Front Synaptic Neurosci. 2023. PMID: 36685084 Free PMC article. Review.
References
-
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387. - PubMed
-
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. - PubMed
-
- Artola A, Singer W. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci. 1993;16:480–487. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
