Diacylglycerol kinase β knockout mice exhibit attention-deficit behavior and an abnormal response on methylphenidate-induced hyperactivity
- PMID: 22590645
- PMCID: PMC3349656
- DOI: 10.1371/journal.pone.0037058
Diacylglycerol kinase β knockout mice exhibit attention-deficit behavior and an abnormal response on methylphenidate-induced hyperactivity
Abstract
Background: Diacylglycerol kinase (DGK) is an enzyme that phosphorylates diacylglycerol to produce phosphatidic acid. DGKβ is one of the subtypes of the DGK family and regulates many intracellular signaling pathways in the central nervous system. Previously, we demonstrated that DGKβ knockout (KO) mice showed various dysfunctions of higher brain function, such as cognitive impairment (with lower spine density), hyperactivity, reduced anxiety, and careless behavior. In the present study, we conducted further tests on DGKβ KO mice in order to investigate the function of DGKβ in the central nervous system, especially in the pathophysiology of attention deficit hyperactivity disorder (ADHD).
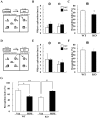
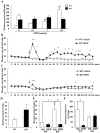
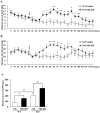
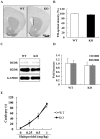
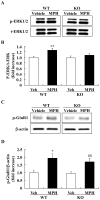
Methodology/principal findings: DGKβ KO mice showed attention-deficit behavior in the object-based attention test and it was ameliorated by methylphenidate (MPH, 30 mg/kg, i.p.). In the open field test, DGKβ KO mice displayed a decreased response to the locomotor stimulating effects of MPH (30 mg/kg, i.p.), but showed a similar response to an N-methyl-d-aspartate (NMDA) receptor antagonist, MK-801 (0.3 mg/kg, i.p.), when compared to WT mice. Examination of the phosphorylation of extracellular signal-regulated kinase (ERK), which is involved in regulation of locomotor activity, indicated that ERK1/2 activation induced by MPH treatment was defective in the striatum of DGKβ KO mice.
Conclusions/significance: These findings suggest that DGKβ KO mice showed attention-deficit and hyperactive phenotype, similar to ADHD. Furthermore, the hyporesponsiveness of DGKβ KO mice to MPH was due to dysregulation of ERK phosphorylation, and that DGKβ has a pivotal involvement in ERK regulation in the striatum.
Conflict of interest statement
Figures
Similar articles
-
Diacylglycerol kinase β knockout mice exhibit lithium-sensitive behavioral abnormalities.PLoS One. 2010 Oct 18;5(10):e13447. doi: 10.1371/journal.pone.0013447. PLoS One. 2010. PMID: 20976192 Free PMC article.
-
The effects of valproate and olanzapine on the abnormal behavior of diacylglycerol kinase β knockout mice.Pharmacol Rep. 2015 Apr;67(2):275-80. doi: 10.1016/j.pharep.2014.10.009. Epub 2014 Oct 29. Pharmacol Rep. 2015. PMID: 25712650
-
Involvement of diacylglycerol kinase β in the spine formation at distal dendrites of striatal medium spiny neurons.Brain Res. 2015 Jan 12;1594:36-45. doi: 10.1016/j.brainres.2014.11.012. Epub 2014 Nov 15. Brain Res. 2015. PMID: 25446448
-
Pharmacogenetics of response to methylphenidate in adult patients with Attention-Deficit/Hyperactivity Disorder (ADHD): a systematic review.Eur Neuropsychopharmacol. 2013 Jun;23(6):555-60. doi: 10.1016/j.euroneuro.2012.05.006. Epub 2012 Jun 17. Eur Neuropsychopharmacol. 2013. PMID: 22709890 Review.
-
Does chirality matter? pharmacodynamics of enantiomers of methylphenidate in patients with attention-deficit/hyperactivity disorder.J Clin Psychopharmacol. 2008 Jun;28(3 Suppl 2):S62-6. doi: 10.1097/JCP.0b013e3181744aa6. J Clin Psychopharmacol. 2008. PMID: 18480679 Review.
Cited by
-
Brinp1(-/-) mice exhibit autism-like behaviour, altered memory, hyperactivity and increased parvalbumin-positive cortical interneuron density.Mol Autism. 2016 Mar 31;7:22. doi: 10.1186/s13229-016-0079-7. eCollection 2016. Mol Autism. 2016. PMID: 27042284 Free PMC article.
-
Diacylglycerol, phosphatidic acid, and their metabolic enzymes in synaptic vesicle recycling.Adv Biol Regul. 2015 Jan;57:147-52. doi: 10.1016/j.jbior.2014.09.010. Epub 2014 Sep 28. Adv Biol Regul. 2015. PMID: 25446883 Free PMC article. Review.
-
Molecular Pathways: Targeting Diacylglycerol Kinase Alpha in Cancer.Clin Cancer Res. 2015 Nov 15;21(22):5008-12. doi: 10.1158/1078-0432.CCR-15-0413. Epub 2015 Sep 29. Clin Cancer Res. 2015. PMID: 26420856 Free PMC article.
-
mRNA expression of diacylglycerol kinase isoforms in insulin-sensitive tissues: effects of obesity and insulin resistance.Physiol Rep. 2015 Apr;3(4):e12372. doi: 10.14814/phy2.12372. Physiol Rep. 2015. PMID: 25847921 Free PMC article.
-
RalA, PLD and mTORC1 Are Required for Kinase-Independent Pathways in DGKβ-Induced Neurite Outgrowth.Biomolecules. 2021 Dec 2;11(12):1814. doi: 10.3390/biom11121814. Biomolecules. 2021. PMID: 34944458 Free PMC article.
References
-
- Rhee SG, Bae YS. Regulation of phosphoinositide-specific phospholipase C isozymes. J Biol Chem. 1997;272:15045–15048. - PubMed
-
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. Faseb J. 1995;9:484–496. - PubMed
-
- Merida I, Avila-Flores A, Merino E. Diacylglycerol kinases: at the hub of cell signalling. Biochem J. 2008;409:1–18. - PubMed
-
- Ghosh S, Strum JC, Sciorra VA, Daniel L, Bell RM. Raf-1 kinase possesses distinct binding domains for phosphatidylserine and phosphatidic acid. Phosphatidic acid regulates the translocation of Raf-1 in 12-O-tetradecanoylphorbol-13-acetate-stimulated Madin-Darby canine kidney cells. J Biol Chem. 1996;271:8472–8480. - PubMed
-
- Kanoh H, Yamada K, Sakane F. Diacylglycerol kinase: a key modulator of signal transduction? Trends Biochem Sci. 1990;15:47–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

