Differential regulation of androgen receptor by PIM-1 kinases via phosphorylation-dependent recruitment of distinct ubiquitin E3 ligases
- PMID: 22584579
- PMCID: PMC3391098
- DOI: 10.1074/jbc.M111.338350
Differential regulation of androgen receptor by PIM-1 kinases via phosphorylation-dependent recruitment of distinct ubiquitin E3 ligases
Abstract
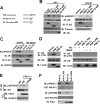
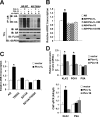
Androgen receptor (AR) plays a pivotal role in prostate cancer. Regulation of AR transcriptional activity by post-translational modifications, such as phosphorylation by multiple kinases, is well documented. Here, we report that two PIM-1 kinase isoforms which are up-regulated during prostate cancer progression, namely PIM-1S and PIM-1L, modulate AR stability and transcriptional activity through differentially phosphorylating AR at serine 213 (Ser-213) and threonine 850 (Thr-850). Although both kinases are capable of interacting with and phosphorylating AR at Ser-213, only PIM-1L could phosphorylate Thr-850. We also showed that PIM-1S induced Ser-213 phosphorylation destabilizes AR by recruiting the ubiquitin E3 ligase Mdm2 and promotes AR degradation in a cell cycle-dependent manner, while PIM-1L-induced Thr-850 phosphorylation stabilizes AR by recruiting the ubiquitin E3 ligase RNF6 and promotes AR-mediated transcription under low-androgen conditions. Furthermore, both PIM-1 isoforms could promote prostate cancer cell growth under low-androgen conditions. Our data suggest that these kinases regulate AR stability and transcriptional activity through recruitment of different functional partners in a phosphorylation-dependent manner. As AR turnover has been previously shown to be critical for cell cycle progression in prostate cancer cells, PIM-1 kinase isoforms may promote prostate cancer cell growth, at least in part, through modulating AR activity via distinct mechanisms.
Figures



Similar articles
-
Implications of ubiquitin ligases in castration-resistant prostate cancer.Curr Opin Oncol. 2015 May;27(3):172-6. doi: 10.1097/CCO.0000000000000178. Curr Opin Oncol. 2015. PMID: 25811345 Free PMC article. Review.
-
p21-Activated kinase 6 (PAK6) inhibits prostate cancer growth via phosphorylation of androgen receptor and tumorigenic E3 ligase murine double minute-2 (Mdm2).J Biol Chem. 2013 Feb 1;288(5):3359-69. doi: 10.1074/jbc.M112.384289. Epub 2012 Nov 6. J Biol Chem. 2013. PMID: 23132866 Free PMC article.
-
Attenuation of androgen receptor-dependent transcription by the serine/threonine kinase Pim-1.Lab Invest. 2003 Sep;83(9):1301-9. doi: 10.1097/01.lab.0000087585.03162.a3. Lab Invest. 2003. PMID: 13679438
-
Phosphorylation of the androgen receptor by PIM1 in hormone refractory prostate cancer.Oncogene. 2013 Aug 22;32(34):3992-4000. doi: 10.1038/onc.2012.412. Epub 2012 Sep 17. Oncogene. 2013. PMID: 22986532 Free PMC article.
-
p27(Kip1) signaling: Transcriptional and post-translational regulation.Int J Biochem Cell Biol. 2015 Nov;68:9-14. doi: 10.1016/j.biocel.2015.08.005. Epub 2015 Aug 14. Int J Biochem Cell Biol. 2015. PMID: 26279144 Review.
Cited by
-
Urolithin A analog inhibits castration-resistant prostate cancer by targeting the androgen receptor and its variant, androgen receptor-variant 7.Front Pharmacol. 2023 Mar 3;14:1137783. doi: 10.3389/fphar.2023.1137783. eCollection 2023. Front Pharmacol. 2023. PMID: 36937838 Free PMC article.
-
Characterizing prostate cancer risk through multi-ancestry genome-wide discovery of 187 novel risk variants.Nat Genet. 2023 Dec;55(12):2065-2074. doi: 10.1038/s41588-023-01534-4. Epub 2023 Nov 9. Nat Genet. 2023. PMID: 37945903 Free PMC article.
-
International Union of Basic and Clinical Pharmacology CXIII: Nuclear Receptor Superfamily-Update 2023.Pharmacol Rev. 2023 Nov;75(6):1233-1318. doi: 10.1124/pharmrev.121.000436. Epub 2023 Aug 16. Pharmacol Rev. 2023. PMID: 37586884 Free PMC article. Review.
-
Conditional transgenic expression of PIM1 kinase in prostate induces inflammation-dependent neoplasia.PLoS One. 2013;8(4):e60277. doi: 10.1371/journal.pone.0060277. Epub 2013 Apr 2. PLoS One. 2013. PMID: 23565217 Free PMC article.
-
Implications of ubiquitin ligases in castration-resistant prostate cancer.Curr Opin Oncol. 2015 May;27(3):172-6. doi: 10.1097/CCO.0000000000000178. Curr Opin Oncol. 2015. PMID: 25811345 Free PMC article. Review.
References
-
- Debes J. D., Tindall D. J. (2004) Mechanisms of androgen-refractory prostate cancer. N. Engl. J. Med. 351, 1488–1490 - PubMed
-
- McPhaul M. J. (2003) Factors that mediate and modulate androgen action. J. Investig. Dermatol. Symp. Proc. 8, 1–5 - PubMed
-
- Culig Z., Hobisch A., Bartsch G., Klocker H. (2000) Androgen receptor–an update of mechanisms of action in prostate cancer. Urol. Res. 28, 211–219 - PubMed
-
- Marcelli M., Cunningham G. R. (1999) Hormonal signaling in prostatic hyperplasia and neoplasia. J. Clin. Endocrinol. Metab. 84, 3463–3468 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

