Serum metabolomic profile as a means to distinguish stage of colorectal cancer
- PMID: 22583555
- PMCID: PMC3506908
- DOI: 10.1186/gm341
Serum metabolomic profile as a means to distinguish stage of colorectal cancer
Abstract
Background: Presently, colorectal cancer (CRC) is staged preoperatively by radiographic tests, and postoperatively by pathological evaluation of available surgical specimens. However, present staging methods do not accurately identify occult metastases. This has a direct effect on clinical management. Early identification of metastases isolated to the liver may enable surgical resection, whereas more disseminated disease may be best treated with palliative chemotherapy.
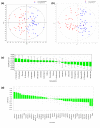
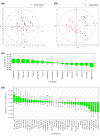
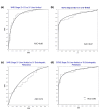
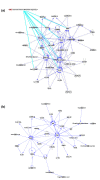
Methods: Sera from 103 patients with colorectal adenocarcinoma treated at the same tertiary cancer center were analyzed by proton nuclear magnetic resonance (1H NMR) spectroscopy and gas chromatography-mass spectroscopy (GC-MS). Metabolic profiling was done using both supervised pattern recognition and orthogonal partial least squares-discriminant analysis (O-PLS-DA) of the most significant metabolites, which enables comparison of the whole sample spectrum between groups. The metabolomic profiles generated from each platform were compared between the following groups: locoregional CRC (N = 42); liver-only metastases (N = 45); and extrahepatic metastases (N = 25).
Results: The serum metabolomic profile associated with locoregional CRC was distinct from that associated with liver-only metastases, based on 1H NMR spectroscopy (P = 5.10 × 10-7) and GC-MS (P = 1.79 × 10-7). Similarly, the serum metabolomic profile differed significantly between patients with liver-only metastases and with extrahepatic metastases. The change in metabolomic profile was most markedly demonstrated on GC-MS (P = 4.75 × 10-5).
Conclusions: In CRC, the serum metabolomic profile changes markedly with metastasis, and site of disease also appears to affect the pattern of circulating metabolites. This novel observation may have clinical utility in enhancing staging accuracy and selecting patients for surgical or medical management. Additional studies are required to determine the sensitivity of this approach to detect subtle or occult metastatic disease.
Figures
Similar articles
-
Translational Metabolomics of Head Injury: Exploring Dysfunctional Cerebral Metabolism with Ex Vivo NMR Spectroscopy-Based Metabolite Quantification.In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 25. In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 25. PMID: 26269925 Free Books & Documents. Review.
-
NMR-based metabolomic techniques identify potential urinary biomarkers for early colorectal cancer detection.Oncotarget. 2017 Nov 11;8(62):105819-105831. doi: 10.18632/oncotarget.22402. eCollection 2017 Dec 1. Oncotarget. 2017. PMID: 29285295 Free PMC article.
-
A quantitative multimodal metabolomic assay for colorectal cancer.BMC Cancer. 2018 Jan 4;18(1):26. doi: 10.1186/s12885-017-3923-z. BMC Cancer. 2018. PMID: 29301511 Free PMC article.
-
[Proton nuclear magnetic resonance spectroscopy recognition of metabolic patterns in fecal extracts for early diagnosis of colorectal cancer].Zhonghua Yu Fang Yi Xue Za Zhi. 2016 Sep 6;50(9):788-793. doi: 10.3760/cma.j.issn.0253-9624.2016.09.008. Zhonghua Yu Fang Yi Xue Za Zhi. 2016. PMID: 27655598 Chinese.
-
The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus.Oncologist. 2012;17(10):1225-39. doi: 10.1634/theoncologist.2012-0121. Epub 2012 Sep 7. Oncologist. 2012. PMID: 22962059 Free PMC article.
Cited by
-
NMR-Based Metabolomics in Cancer Research.Adv Exp Med Biol. 2021;1280:201-218. doi: 10.1007/978-3-030-51652-9_14. Adv Exp Med Biol. 2021. PMID: 33791984
-
Molecular portraits: the evolution of the concept of transcriptome-based cancer signatures.Brief Bioinform. 2015 Nov;16(6):1000-7. doi: 10.1093/bib/bbv013. Epub 2015 Mar 31. Brief Bioinform. 2015. PMID: 25832647 Free PMC article. Review.
-
Temporal characterization of serum metabolite signatures in lung cancer patients undergoing treatment.Metabolomics. 2016;12:58. doi: 10.1007/s11306-016-0961-5. Epub 2016 Feb 27. Metabolomics. 2016. PMID: 27073350 Free PMC article.
-
Early detection of treatment futility in patients with metastatic colorectal cancer.Oncotarget. 2022 Jan 7;13:61-72. doi: 10.18632/oncotarget.28165. eCollection 2022. Oncotarget. 2022. PMID: 35028011 Free PMC article. Clinical Trial.
-
Metabolomics profile in gastrointestinal cancers: Update and future perspectives.World J Gastroenterol. 2020 May 28;26(20):2514-2532. doi: 10.3748/wjg.v26.i20.2514. World J Gastroenterol. 2020. PMID: 32523308 Free PMC article. Review.
References
-
- Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, Curley SA, Loyer EM, Muratore A, Mentha G, Capussotti L, Vauthey JN. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715. doi: 10.1097/01.sla.0000160703.75808.7d. - DOI - PMC - PubMed
-
- Bathe OF, Ernst S, Sutherland FR, Dixon E, Butts C, Bigam D, Holland D, Porter GA, Koppel J, Dowden S. A phase II experience with neoadjuvant irinotecan (CPT-11), 5-fluorouracil (5-FU) and leucovorin (LV) for colorectal liver metastases. BMC Cancer. 2009;9:156. doi: 10.1186/1471-2407-9-156. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

