Calpains, mitochondria, and apoptosis
- PMID: 22581845
- PMCID: PMC3444233
- DOI: 10.1093/cvr/cvs163
Calpains, mitochondria, and apoptosis
Abstract
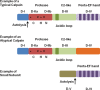
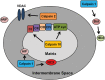
Mitochondrial activity is critical for efficient function of the cardiovascular system. In response to cardiovascular injury, mitochondrial dysfunction occurs and can lead to apoptosis and necrosis. Calpains are a 15-member family of Ca(2+)-activated cysteine proteases localized to the cytosol and mitochondria, and several have been shown to regulate apoptosis and necrosis. For example, in endothelial cells, Ca(2+) overload causes mitochondrial calpain 1 cleavage of the Na(+)/Ca(2+) exchanger leading to mitochondrial Ca(2+) accumulation. Also, activated calpain 1 cleaves Bid, inducing cytochrome c release and apoptosis. In renal cells, calpains 1 and 2 promote apoptosis and necrosis by cleaving cytoskeletal proteins, which increases plasma membrane permeability and cleavage of caspases. Calpain 10 cleaves electron transport chain proteins, causing decreased mitochondrial respiration and excessive activation, or inhibition of calpain 10 activity induces mitochondrial dysfunction and apoptosis. In cardiomyocytes, calpain 1 activates caspase 3 and poly-ADP ribose polymerase during tumour necrosis factor-α-induced apoptosis, and calpain 1 cleaves apoptosis-inducing factor after Ca(2+) overload. Many of these observations have been elucidated with calpain inhibitors, but most calpain inhibitors are not specific for calpains or a specific calpain family member, creating more questions. The following review will discuss how calpains affect mitochondrial function and apoptosis within the cardiovascular system.
Figures
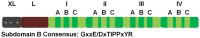
Similar articles
-
EspC, an Autotransporter Protein Secreted by Enteropathogenic Escherichia coli, Causes Apoptosis and Necrosis through Caspase and Calpain Activation, Including Direct Procaspase-3 Cleavage.mBio. 2016 Jun 21;7(3):e00479-16. doi: 10.1128/mBio.00479-16. mBio. 2016. PMID: 27329750 Free PMC article.
-
Group B Streptococcus induces macrophage apoptosis by calpain activation.J Immunol. 2006 Jun 15;176(12):7542-56. doi: 10.4049/jimmunol.176.12.7542. J Immunol. 2006. PMID: 16751401
-
The role of calpain in oncotic cell death.Annu Rev Pharmacol Toxicol. 2004;44:349-70. doi: 10.1146/annurev.pharmtox.44.101802.121804. Annu Rev Pharmacol Toxicol. 2004. PMID: 14744250 Review.
-
Mitochondrial m-calpain plays a role in the release of truncated apoptosis-inducing factor from the mitochondria.Biochim Biophys Acta. 2009 Dec;1793(12):1848-59. doi: 10.1016/j.bbamcr.2009.10.002. Epub 2009 Oct 13. Biochim Biophys Acta. 2009. PMID: 19833151
-
Proteases in renal cell death: calpains mediate cell death produced by diverse toxicants.Ren Fail. 1998 Sep;20(5):679-86. doi: 10.3109/08860229809045162. Ren Fail. 1998. PMID: 9768434 Review.
Cited by
-
Myocardial injury, troponin release, and cardiomyocyte death in brief ischemia, failure, and ventricular remodeling.Am J Physiol Heart Circ Physiol. 2022 Jul 1;323(1):H1-H15. doi: 10.1152/ajpheart.00093.2022. Epub 2022 May 13. Am J Physiol Heart Circ Physiol. 2022. PMID: 35559722 Free PMC article. Review.
-
The NO/ONOO-cycle as the central cause of heart failure.Int J Mol Sci. 2013 Nov 13;14(11):22274-330. doi: 10.3390/ijms141122274. Int J Mol Sci. 2013. PMID: 24232452 Free PMC article. Review.
-
Aberrant mechanical loading induces annulus fibrosus cells apoptosis in intervertebral disc degeneration via mechanosensitive ion channel Piezo1.Arthritis Res Ther. 2023 Jul 7;25(1):117. doi: 10.1186/s13075-023-03093-9. Arthritis Res Ther. 2023. PMID: 37420255 Free PMC article.
-
Astragaloside IV attenuates inflammatory response mediated by NLRP-3/calpain-1 is involved in the development of pulmonary hypertension.J Cell Mol Med. 2021 Jan;25(1):586-590. doi: 10.1111/jcmm.15671. Epub 2020 Dec 8. J Cell Mol Med. 2021. PMID: 33295020 Free PMC article.
-
Loss-of-function mutations in CAST cause peeling skin, leukonychia, acral punctate keratoses, cheilitis, and knuckle pads.Am J Hum Genet. 2015 Mar 5;96(3):440-7. doi: 10.1016/j.ajhg.2014.12.026. Epub 2015 Feb 12. Am J Hum Genet. 2015. PMID: 25683118 Free PMC article.
References
-
- Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. - PubMed
-
- Dayton WR, Reville WJ, Goll DE, Stromer MH. A calcium(2+) ion-activated protease possibly involved in myofibrillar protein turnover. Partial characterization of the purified enzyme. Biochemistry. 1976;15:2159–2167. - PubMed
-
- Dayton WR, Goll DE, Zeece MG, Robson RM, Reville WJ. A Ca2+ ion-activated protease possibly involved in myofibrillar protein turnover. Purification from porcine muscle. Biochemistry. 1976;15:2150–2158. - PubMed
-
- Futai E, Kubo T, Sorimachi H, Suzuki K, Maeda T. Molecular cloning of PalBH, a mammalian homologue of the Aspergillus atypical calpain PalB. Biochim Biophys Acta. 2001;1517:316–319. - PubMed
-
- Lee H-J, Tomioka S, Kinbara K, Masumoto H, Jeong S-Y, Sorimachi H, et al. Characterization of a human digestive tract-specific calpain, nCL-4, expressed in the baculovirus system. Arch Biochem Biophys. 1999;362:22–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

