Astrocytes in the rat medial amygdala are responsive to adult androgens
- PMID: 22581688
- PMCID: PMC4209966
- DOI: 10.1002/cne.23061
Astrocytes in the rat medial amygdala are responsive to adult androgens
Abstract
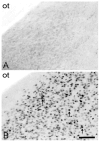
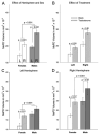
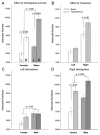
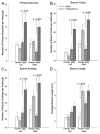
The posterodorsal medial amygdala (MePD) exhibits numerous sex differences including differences in volume and in the number and morphology of neurons and astroctyes. In adulthood, gonadal hormones, including both androgens and estrogens, have been shown to play a role in maintaining the masculine character of many of these sex differences, but whether adult gonadal hormones maintain the increased number and complexity of astrocytes in the male MePD was unknown. To answer this question we examined astrocytes in the MePD of male and female Long Evans rats that were gonadectomized as adults and treated for 30 days with either testosterone or a control treatment. At the end of treatment brains were collected and immunostained for glial fibrillary acidic protein. Stereological analysis revealed that adult androgen levels influenced the number and complexity of astrocytes in the MePD of both sexes, but the specific effects of androgens were different in males and females. However, sex differences in the number and complexity of adult astrocytes persisted even in the absence of gonadal hormones in adulthood, suggesting that androgens also act earlier in life to determine these adult sex differences. Using immunofluorescence and confocal microscopy, we found robust androgen receptor immunostaining in a subpopulation of MePD astrocytes, suggesting that testosterone may act directly on MePD astrocytes to influence their structure and function.
Copyright © 2012 Wiley Periodicals, Inc.
Figures

Similar articles
-
Sexual dimorphism in neuronal number of the posterodorsal medial amygdala is independent of circulating androgens and regional volume in adult rats.J Comp Neurol. 2008 Feb 10;506(5):851-9. doi: 10.1002/cne.21536. J Comp Neurol. 2008. PMID: 18076082
-
Sex differences and laterality in astrocyte number and complexity in the adult rat medial amygdala.J Comp Neurol. 2008 Dec 10;511(5):599-609. doi: 10.1002/cne.21859. J Comp Neurol. 2008. PMID: 18853427 Free PMC article.
-
Sex and laterality differences in medial amygdala neurons and astrocytes of adult mice.J Comp Neurol. 2016 Aug 15;524(12):2492-502. doi: 10.1002/cne.23964. Epub 2016 Feb 3. J Comp Neurol. 2016. PMID: 26780286 Free PMC article.
-
Steroid hormone masculinization of neural structure in rats: a tale of two nuclei.Physiol Behav. 2004 Nov 15;83(2):271-7. doi: 10.1016/j.physbeh.2004.08.016. Physiol Behav. 2004. PMID: 15488544 Review.
-
Steroid-dependent plasticity in the medial amygdala.Neuroscience. 2006;138(3):997-1005. doi: 10.1016/j.neuroscience.2005.06.018. Epub 2005 Dec 5. Neuroscience. 2006. PMID: 16330154 Review.
Cited by
-
Androgen receptors in muscle fibers induce rapid loss of force but not mass: implications for spinal bulbar muscular atrophy.Muscle Nerve. 2013 Jun;47(6):823-34. doi: 10.1002/mus.23813. Epub 2013 Apr 30. Muscle Nerve. 2013. PMID: 23629944 Free PMC article.
-
The organizing actions of adolescent gonadal steroid hormones on brain and behavioral development.Neurosci Biobehav Rev. 2016 Nov;70:148-158. doi: 10.1016/j.neubiorev.2016.07.036. Epub 2016 Aug 4. Neurosci Biobehav Rev. 2016. PMID: 27497718 Free PMC article. Review.
-
Anxiety in transition: Neuroendocrine mechanisms supporting the development of anxiety pathology in adolescence and young adulthood.Front Neuroendocrinol. 2019 Oct;55:100791. doi: 10.1016/j.yfrne.2019.100791. Epub 2019 Sep 19. Front Neuroendocrinol. 2019. PMID: 31542287 Free PMC article. Review.
-
Prenatal bisphenol A (BPA) exposure alters the transcriptome of the neonate rat amygdala in a sex-specific manner: a CLARITY-BPA consortium study.Neurotoxicology. 2018 Mar;65:207-220. doi: 10.1016/j.neuro.2017.10.005. Epub 2017 Oct 28. Neurotoxicology. 2018. PMID: 29097150 Free PMC article.
-
Immature excitatory neurons in the amygdala come of age during puberty.Dev Cogn Neurosci. 2022 Aug;56:101133. doi: 10.1016/j.dcn.2022.101133. Epub 2022 Jul 10. Dev Cogn Neurosci. 2022. PMID: 35841648 Free PMC article.
References
-
- Adamec RE. Amygdala kindling and anxiety in the rat. Neuroreport. 1990;1:255–258. - PubMed
-
- Adolphs R, Tranel D. Amygdala damage impairs emotion recognition from scenes only when they contain facial expressions. Neuropsychologia. 2003;41:1281–1289. - PubMed
-
- Adolphs R, Baron-Cohen S, Tranel D. Impaired recognition of social emotions following amygdala damage. J Cogn Neurosci. 2002;14:1264–1274. - PubMed
-
- Agapova OA, Malone PE, Hernandez MR. A neuroactive steroid 5alpha-androstane-3alpha,17beta-diol regulates androgen receptor level in astrocytes. J Neurochem. 2006;98:355–363. - PubMed
-
- Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 2001;892:255–262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

