Toll-like receptor 4 deficiency decreases atherosclerosis but does not protect against inflammation in obese low-density lipoprotein receptor-deficient mice
- PMID: 22580897
- PMCID: PMC3748807
- DOI: 10.1161/ATVBAHA.112.249847
Toll-like receptor 4 deficiency decreases atherosclerosis but does not protect against inflammation in obese low-density lipoprotein receptor-deficient mice
Abstract
Objective: Obesity is associated with insulin resistance, chronic low-grade inflammation, and atherosclerosis. Toll-like receptor 4 (TLR4) participates in the cross talk between inflammation and insulin resistance, being activated by both lipopolysaccharide and saturated fatty acids. The present study was undertaken to determine whether TLR4 deficiency has a protective role in inflammation, insulin resistance, and atherosclerosis induced by a diabetogenic diet.
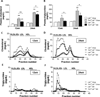
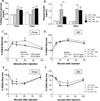
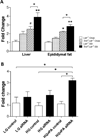
Methods and results: TLR4 and low-density lipoprotein (LDL) receptor double knockout mice and LDL receptor-deficient mice were fed either a normal chow or a diabetogenic diet for 24 weeks. TLR4 and LDL receptor double knockout mice fed a diabetogenic diet showed improved plasma cholesterol and triglyceride levels but developed obesity, hyperinsulinemia, and glucose intolerance equivalent to obese LDL receptor-deficient mice. Adipocyte hypertrophy, macrophage accumulation, and local inflammation were not attenuated in intraabdominal adipose tissue in TLR4 and LDL receptor double knockout mice. However, TLR4 deficiency led to markedly decreased atherosclerosis in obese TLR4 and LDL receptor double knockout mice. Compensatory upregulation of TLR2 expression was observed both in obese TLR4-deficient mice and in palmitate-treated TLR4-silenced 3T3-L1 adipocytes.
Conclusions: TLR4 deficiency decreases atherosclerosis without affecting obesity-induced inflammation and insulin resistance in LDL receptor-deficient mice. Alternative pathways may be responsible for adipose tissue macrophage infiltration and insulin resistance that occurs in obesity.
Figures



Similar articles
-
Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese LDL receptor-deficient mice.Arterioscler Thromb Vasc Biol. 2008 Apr;28(4):685-91. doi: 10.1161/ATVBAHA.107.157685. Epub 2008 Jan 31. Arterioscler Thromb Vasc Biol. 2008. PMID: 18239153 Free PMC article.
-
TLR4 antagonist attenuates atherogenesis in LDL receptor-deficient mice with diet-induced type 2 diabetes.Immunobiology. 2015 Nov;220(11):1246-54. doi: 10.1016/j.imbio.2015.06.016. Epub 2015 Jun 30. Immunobiology. 2015. PMID: 26162692 Free PMC article.
-
S100A9 differentially modifies phenotypic states of neutrophils, macrophages, and dendritic cells: implications for atherosclerosis and adipose tissue inflammation.Circulation. 2011 Mar 22;123(11):1216-26. doi: 10.1161/CIRCULATIONAHA.110.985523. Epub 2011 Mar 7. Circulation. 2011. PMID: 21382888 Free PMC article.
-
Relations between metabolic syndrome, oxidative stress and inflammation and cardiovascular disease.Verh K Acad Geneeskd Belg. 2008;70(3):193-219. Verh K Acad Geneeskd Belg. 2008. PMID: 18669160 Review.
-
Impacts of the apoptosis inhibitor of macrophage (AIM) on obesity-associated inflammatory diseases.Semin Immunopathol. 2014 Jan;36(1):3-12. doi: 10.1007/s00281-013-0405-5. Epub 2013 Nov 27. Semin Immunopathol. 2014. PMID: 24281248 Free PMC article. Review.
Cited by
-
Lipids and the endothelium: bidirectional interactions.Curr Atheroscler Rep. 2013 Nov;15(11):365. doi: 10.1007/s11883-013-0365-1. Curr Atheroscler Rep. 2013. PMID: 24037142 Free PMC article. Review.
-
Toll-like receptor-4 mediated inflammation is involved in the cardiometabolic alterations induced by intermittent hypoxia.Mediators Inflamm. 2015;2015:620258. doi: 10.1155/2015/620258. Epub 2015 Mar 19. Mediators Inflamm. 2015. PMID: 25873766 Free PMC article. Review.
-
Molecular Mechanisms Underlying Obesity-Induced Hypothalamic Inflammation and Insulin Resistance: Pivotal Role of Resistin/TLR4 Pathways.Front Endocrinol (Lausanne). 2019 Mar 8;10:140. doi: 10.3389/fendo.2019.00140. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 30906281 Free PMC article. Review.
-
Eotaxin-2 increased toll-like receptor 4 expression in endothelial cells in vitro and exacerbates high-cholesterol diet-induced atherogenesis in vivo.Am J Transl Res. 2016 Dec 15;8(12):5338-5353. eCollection 2016. Am J Transl Res. 2016. PMID: 28078007 Free PMC article.
-
Toll-like receptor 7 deficiency protects apolipoprotein E-deficient mice from diet-induced atherosclerosis.Sci Rep. 2017 Apr 12;7(1):847. doi: 10.1038/s41598-017-00977-0. Sci Rep. 2017. PMID: 28405010 Free PMC article.
References
-
- Shoelson SE, Herrero L, Naaz A. Obesity, inflammation and insulin resistance. Gastroenterology. 2007;132:2169–2180. - PubMed
-
- Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. - PubMed
-
- Pouliot MC, Després JP, Nadeau A, Moorjani S, Prud'Homme D, Lupien PJ, Tremblay A, Bouchard C. Visceral obesity in men: associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes. 1992;41:826–834. - PubMed
-
- Carey VJ, Walters EE, Colditz GA, Solomon CG, Willett WC, Rosner BA, Speizer FE, Manson JE. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women: the nurse's health study. Am J Epidemiol. 1997;145:614–619. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

