The KDEL receptor couples to Gαq/11 to activate Src kinases and regulate transport through the Golgi
- PMID: 22580821
- PMCID: PMC3395092
- DOI: 10.1038/emboj.2012.134
The KDEL receptor couples to Gαq/11 to activate Src kinases and regulate transport through the Golgi
Abstract
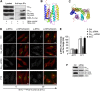
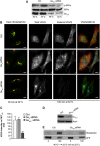
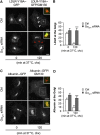
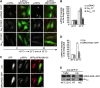
Membrane trafficking involves large fluxes of cargo and membrane across separate compartments. These fluxes must be regulated by control systems to maintain homoeostasis. While control systems for other key functions such as protein folding or the cell cycle are well known, the mechanisms that control secretory transport are poorly understood. We have previously described a signalling circuit operating at the Golgi complex that regulates intra-Golgi trafficking and is initiated by the KDEL receptor (KDEL-R), a protein previously known to mediate protein recycling from the Golgi to the endoplasmic reticulum (ER). Here, we investigated the KDEL-R signalling mechanism. We show that the KDEL-R is predicted to fold like a G-protein-coupled receptor (GPCR), and that it binds and activates the heterotrimeric signalling G-protein Gα(q/11) which, in turn, regulates transport through the Golgi complex. These findings reveal an unexpected GPCR-like mode of action of the KDEL-R and shed light on a core molecular control mechanism of intra-Golgi traffic.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
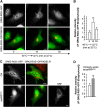
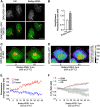
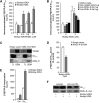
Similar articles
-
Src regulates Golgi structure and KDEL receptor-dependent retrograde transport to the endoplasmic reticulum.J Biol Chem. 2003 Nov 21;278(47):46601-6. doi: 10.1074/jbc.M302221200. Epub 2003 Sep 15. J Biol Chem. 2003. PMID: 12975382
-
A traffic-activated Golgi-based signalling circuit coordinates the secretory pathway.Nat Cell Biol. 2008 Aug;10(8):912-22. doi: 10.1038/ncb1751. Epub 2008 Jul 20. Nat Cell Biol. 2008. PMID: 18641641
-
Prohibitin: A Novel Molecular Player in KDEL Receptor Signalling.Biomed Res Int. 2015;2015:319454. doi: 10.1155/2015/319454. Epub 2015 May 10. Biomed Res Int. 2015. PMID: 26064897 Free PMC article.
-
Regulation of Golgi signaling and trafficking by the KDEL receptor.Histochem Cell Biol. 2013 Oct;140(4):395-405. doi: 10.1007/s00418-013-1130-9. Epub 2013 Jul 20. Histochem Cell Biol. 2013. PMID: 23873287 Review.
-
Pathogenic Effects of Impaired Retrieval between the Endoplasmic Reticulum and Golgi Complex.Int J Mol Sci. 2019 Nov 9;20(22):5614. doi: 10.3390/ijms20225614. Int J Mol Sci. 2019. PMID: 31717602 Free PMC article. Review.
Cited by
-
A Golgi-based KDELR-dependent signalling pathway controls extracellular matrix degradation.Oncotarget. 2015 Feb 20;6(5):3375-93. doi: 10.18632/oncotarget.3270. Oncotarget. 2015. PMID: 25682866 Free PMC article.
-
The KDEL receptor signalling cascade targets focal adhesion kinase on focal adhesions and invadopodia.Oncotarget. 2017 Dec 19;9(12):10228-10246. doi: 10.18632/oncotarget.23421. eCollection 2018 Feb 13. Oncotarget. 2017. PMID: 29535802 Free PMC article.
-
Constitutive phospholipid scramblase activity of a G protein-coupled receptor.Nat Commun. 2014 Oct 8;5:5115. doi: 10.1038/ncomms6115. Nat Commun. 2014. PMID: 25296113 Free PMC article.
-
Heterotrimeric G protein signaling via GIV/Girdin: Breaking the rules of engagement, space, and time.Bioessays. 2016 Apr;38(4):379-93. doi: 10.1002/bies.201500133. Epub 2016 Feb 16. Bioessays. 2016. PMID: 26879989 Free PMC article. Review.
-
Accommodation of large cargo within Golgi cisternae.Histochem Cell Biol. 2013 Sep;140(3):261-9. doi: 10.1007/s00418-013-1120-y. Epub 2013 Jul 3. Histochem Cell Biol. 2013. PMID: 23821163 Free PMC article. Review.
References
-
- Alberts B, Johnson A, Lewis J, Raff M, Roberts KPW (2002) Molecular Biology of the Cell 4th ednNew York: Garland Science
-
- Balch WE, Dunphy WG, Braell WA, Rothman JE (1984) Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell 39: 2 Part 1405–416 - PubMed
-
- Berman DM, Kozasa T, Gilman AG (1996) The GTPase-activating protein RGS4 stabilizes the transition state for nucleotide hydrolysis. J Biol Chem 271: 27209–27212 - PubMed
-
- Bonazzi M, Spano S, Turacchio G, Cericola C, Valente C, Colanzi A, Kweon HS, Hsu VW, Polishchuck EV, Polishchuck RS, Sallese M, Pulvirenti T, Corda D, Luini A (2005) CtBP3/BARS drives membrane fission in dynamin-independent transport pathways. Nat Cell Biol 7: 570–580 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

