CB₂ cannabinoid receptors inhibit synaptic transmission when expressed in cultured autaptic neurons
- PMID: 22579668
- PMCID: PMC3392362
- DOI: 10.1016/j.neuropharm.2012.04.024
CB₂ cannabinoid receptors inhibit synaptic transmission when expressed in cultured autaptic neurons
Abstract
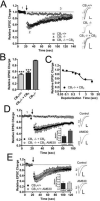
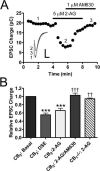
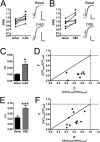
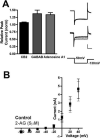
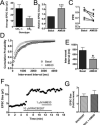
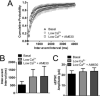
The role of CB₂ in the central nervous system, particularly in neurons, has generated much controversy. Fueling the controversy are imperfect tools, which have made conclusive identification of CB₂ expressing neurons problematic. Imprecise localization of CB₂ has made it difficult to determine its function in neurons. Here we avoid the localization controversy and directly address the question if CB₂ can modulate neurotransmission. CB₂ was expressed in excitatory hippocampal autaptic neurons obtained from CB₁ null mice. Whole-cell patch clamp recordings were made from these neurons to determine the effects of CB₂ on short-term synaptic plasticity. CB₂ expression restored depolarization induced suppression of excitation to these neurons, which was lost following genetic ablation of CB₁. The endocannabinoid 2-arachidonylglycerol (2-AG) mimicked the effects of depolarization in CB₂ expressing neurons. Interestingly, ongoing basal production of 2-AG resulted in constitutive activation of CB₂, causing a tonic inhibition of neurotransmission that was relieved by the CB₂ antagonist AM630 or the diacylglycerol lipase inhibitor RHC80267. Through immunocytochemistry and analysis of spontaneous EPSCs, paired pulse ratios and coefficients of variation we determined that CB₂ exerts its function at a presynaptic site of action, likely through inhibition of voltage gated calcium channels. Therefore CB₂ expressed in neurons effectively mimics the actions of CB₁. Thus neuronal CB₂ is well suited to integrate into conventional neuronal endocannabinoid signaling processes, with its specific role determined by its unique and highly inducible expression profile.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Monoacylglycerol lipase limits the duration of endocannabinoid-mediated depolarization-induced suppression of excitation in autaptic hippocampal neurons.Mol Pharmacol. 2009 Dec;76(6):1220-7. doi: 10.1124/mol.109.059030. Epub 2009 Sep 18. Mol Pharmacol. 2009. PMID: 19767452 Free PMC article.
-
Cannabinoid signaling in inhibitory autaptic hippocampal neurons.Neuroscience. 2009 Sep 29;163(1):190-201. doi: 10.1016/j.neuroscience.2009.06.004. Epub 2009 Jun 6. Neuroscience. 2009. PMID: 19501632 Free PMC article.
-
Diacylglycerol lipaseα (DAGLα) and DAGLβ cooperatively regulate the production of 2-arachidonoyl glycerol in autaptic hippocampal neurons.Mol Pharmacol. 2013 Aug;84(2):296-302. doi: 10.1124/mol.113.085217. Epub 2013 Jun 7. Mol Pharmacol. 2013. PMID: 23748223 Free PMC article.
-
Why do cannabinoid receptors have more than one endogenous ligand?Philos Trans R Soc Lond B Biol Sci. 2012 Dec 5;367(1607):3216-28. doi: 10.1098/rstb.2011.0382. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 23108541 Free PMC article. Review.
-
The evolution and comparative neurobiology of endocannabinoid signalling.Philos Trans R Soc Lond B Biol Sci. 2012 Dec 5;367(1607):3201-15. doi: 10.1098/rstb.2011.0394. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 23108540 Free PMC article. Review.
Cited by
-
Bilateral changes of cannabinoid receptor type 2 protein and mRNA in the dorsal root ganglia of a rat neuropathic pain model.J Histochem Cytochem. 2013 Jul;61(7):529-47. doi: 10.1369/0022155413491269. Epub 2013 May 8. J Histochem Cytochem. 2013. PMID: 23657829 Free PMC article.
-
Detection of cannabinoid receptors CB1 and CB2 within basal ganglia output neurons in macaques: changes following experimental parkinsonism.Brain Struct Funct. 2015 Sep;220(5):2721-38. doi: 10.1007/s00429-014-0823-8. Epub 2014 Jun 28. Brain Struct Funct. 2015. PMID: 24972960 Free PMC article.
-
The Endocannabinoid/Cannabinoid Receptor 2 System Protects Against Cisplatin-Induced Hearing Loss.Front Cell Neurosci. 2018 Aug 21;12:271. doi: 10.3389/fncel.2018.00271. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30186120 Free PMC article.
-
CB2 Cannabinoid receptors as a therapeutic target-what does the future hold?Mol Pharmacol. 2014 Oct;86(4):430-7. doi: 10.1124/mol.114.094649. Epub 2014 Aug 8. Mol Pharmacol. 2014. PMID: 25106425 Free PMC article. Review.
-
Role of Cannabinoid CB2 Receptor in Alcohol Use Disorders: From Animal to Human Studies.Int J Mol Sci. 2022 May 25;23(11):5908. doi: 10.3390/ijms23115908. Int J Mol Sci. 2022. PMID: 35682586 Free PMC article. Review.
References
-
- Ashton JC, Rahman RM, Nair SM, Sutherland BA, Glass M, Appleton I. Cerebral hypoxia-ischemia and middle cerebral artery occlusion induce expression of the cannabinoid CB2 receptor in the brain. Neurosci. Lett. 2007;412:114–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

