Allostery in the Hsp70 chaperone proteins
- PMID: 22576356
- PMCID: PMC3623542
- DOI: 10.1007/128_2012_323
Allostery in the Hsp70 chaperone proteins
Abstract
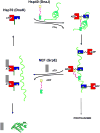
Heat shock 70-kDa (Hsp70) chaperones are essential to in vivo protein folding, protein transport, and protein re-folding. They carry out these activities using repeated cycles of binding and release of client proteins. This process is under allosteric control of nucleotide binding and hydrolysis. X-ray crystallography, NMR spectroscopy, and other biophysical techniques have contributed much to the understanding of the allosteric mechanism linking these activities and the effect of co-chaperones on this mechanism. In this chapter these findings are critically reviewed. Studies on the allosteric mechanisms of Hsp70 have gained enhanced urgency, as recent studies have implicated this chaperone as a potential drug target in diseases such as Alzheimer's and cancer. Recent approaches to combat these diseases through interference with the Hsp70 allosteric mechanism are discussed.
Figures













Similar articles
-
Conformational equilibria in allosteric control of Hsp70 chaperones.Mol Cell. 2021 Oct 7;81(19):3919-3933.e7. doi: 10.1016/j.molcel.2021.07.039. Epub 2021 Aug 27. Mol Cell. 2021. PMID: 34453889 Free PMC article.
-
Pathways of allosteric regulation in Hsp70 chaperones.Nat Commun. 2015 Sep 18;6:8308. doi: 10.1038/ncomms9308. Nat Commun. 2015. PMID: 26383706 Free PMC article.
-
Capturing intrinsic nanomechanics of allostery.Biophys J. 2022 Dec 6;121(23):4415-4416. doi: 10.1016/j.bpj.2022.10.037. Epub 2022 Oct 29. Biophys J. 2022. PMID: 36815705 Free PMC article.
-
Mechanisms of the Hsp70 chaperone system.Biochem Cell Biol. 2010 Apr;88(2):291-300. doi: 10.1139/o09-175. Biochem Cell Biol. 2010. PMID: 20453930 Free PMC article. Review.
-
Hsp70 chaperone dynamics and molecular mechanism.Trends Biochem Sci. 2013 Oct;38(10):507-14. doi: 10.1016/j.tibs.2013.08.001. Epub 2013 Sep 5. Trends Biochem Sci. 2013. PMID: 24012426 Review.
Cited by
-
Cytosolic protein quality control machinery: Interactions of Hsp70 with a network of co-chaperones and substrates.Exp Biol Med (Maywood). 2021 Jun;246(12):1419-1434. doi: 10.1177/1535370221999812. Epub 2021 Mar 17. Exp Biol Med (Maywood). 2021. PMID: 33730888 Free PMC article. Review.
-
Conditional disorder in chaperone action.Trends Biochem Sci. 2012 Dec;37(12):517-25. doi: 10.1016/j.tibs.2012.08.006. Epub 2012 Sep 24. Trends Biochem Sci. 2012. PMID: 23018052 Free PMC article. Review.
-
Abrogating the Interaction Between p53 and Mortalin (Grp75/HSPA9/mtHsp70) for Cancer Therapy: The Story so far.Front Cell Dev Biol. 2022 Apr 14;10:879632. doi: 10.3389/fcell.2022.879632. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35493098 Free PMC article. Review.
-
Chaperone machines for protein folding, unfolding and disaggregation.Nat Rev Mol Cell Biol. 2013 Oct;14(10):630-42. doi: 10.1038/nrm3658. Epub 2013 Sep 12. Nat Rev Mol Cell Biol. 2013. PMID: 24026055 Free PMC article. Review.
-
Heat Shock Response and Heat Shock Proteins: Current Understanding and Future Opportunities in Human Diseases.Int J Mol Sci. 2024 Apr 10;25(8):4209. doi: 10.3390/ijms25084209. Int J Mol Sci. 2024. PMID: 38673794 Free PMC article. Review.
References
-
- Hightower LE. Heat-Shock, Stress Proteins, Chaperones, and Proteotoxicity. Cell. 1991;66:191–197. - PubMed
-
- Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–51. - PubMed
-
- Sfatos CD, Gutin AM, Abkevich VI, Shakhnovich EI. Simulations of chaperone-assisted folding. Biochemistry. 1996;35:334–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

