Antitumoral activity of parvovirus-mediated IL-2 and MCP-3/CCL7 delivery into human pancreatic cancer: implication of leucocyte recruitment
- PMID: 22576056
- PMCID: PMC11028688
- DOI: 10.1007/s00262-012-1279-4
Antitumoral activity of parvovirus-mediated IL-2 and MCP-3/CCL7 delivery into human pancreatic cancer: implication of leucocyte recruitment
Abstract
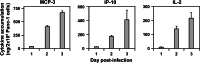
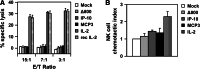
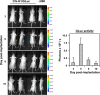
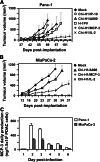
Pancreatic ductal adenocarcinoma (PDAC) represents the fourth leading cause of cancer-related death in western countries. The patients are often diagnosed in advanced metastatic stages, and the prognosis remains extremely poor with an overall 5-year survival rate less than 5 %. Currently, novel therapeutic strategies are being pursued to combat PDAC, including oncolytic viruses, either in their natural forms or armed with immunostimulatory molecules. Natural killer cells are critical players against tumours and infected cells. Recently, we showed that IL-2-activated human NK cells displayed killing activity against PDAC cells, which could further be enhanced through the infection of PDAC cells with the rodent parvovirus H-1PV. In this study, the therapeutic efficacy of parvovirus-mediated delivery of three distinct cyto/chemokines (Il-2, MCP-3/CCL7 and IP-10/CXCL10) was evaluated in xenograft models of human PDAC. We show here that activated NK and monocytic cells were found to be recruited by PDAC tumours upon infection with parvoviruses armed with IL-2 or the chemokine MCP-3/CCL7, resulting in a strong anti-tumour response.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Enhancement of NK cell antitumor responses using an oncolytic parvovirus.Int J Cancer. 2011 Feb 15;128(4):908-19. doi: 10.1002/ijc.25415. Int J Cancer. 2011. PMID: 20473905
-
NK-cell-dependent killing of colon carcinoma cells is mediated by natural cytotoxicity receptors (NCRs) and stimulated by parvovirus infection of target cells.BMC Cancer. 2013 Jul 31;13:367. doi: 10.1186/1471-2407-13-367. BMC Cancer. 2013. PMID: 23902851 Free PMC article.
-
Interferon γ improves the vaccination potential of oncolytic parvovirus H-1PV for the treatment of peritoneal carcinomatosis in pancreatic cancer.Cancer Biol Ther. 2011 Nov 15;12(10):888-95. doi: 10.4161/cbt.12.10.17678. Epub 2011 Nov 15. Cancer Biol Ther. 2011. PMID: 22024742 Free PMC article.
-
The Present Status of Immuno-Oncolytic Viruses in the Treatment of Pancreatic Cancer.Viruses. 2020 Nov 17;12(11):1318. doi: 10.3390/v12111318. Viruses. 2020. PMID: 33213031 Free PMC article. Review.
-
Oncolytic virotherapy for pancreatic ductal adenocarcinoma: A glimmer of hope after years of disappointment?Cytokine Growth Factor Rev. 2020 Dec;56:141-148. doi: 10.1016/j.cytogfr.2020.07.015. Epub 2020 Aug 8. Cytokine Growth Factor Rev. 2020. PMID: 32859494 Review.
Cited by
-
The Prolactin Inducible Protein Modulates Antitumor Immune Responses and Metastasis in a Mouse Model of Triple Negative Breast Cancer.Front Oncol. 2021 Mar 12;11:639859. doi: 10.3389/fonc.2021.639859. eCollection 2021. Front Oncol. 2021. PMID: 33777801 Free PMC article.
-
The anti-cancerous activity of recombinant trichosanthin on prostate cancer cell PC3.Biol Res. 2016 Mar 25;49:21. doi: 10.1186/s40659-016-0081-8. Biol Res. 2016. PMID: 27015938 Free PMC article.
-
Trial Watch-Oncolytic viruses and cancer therapy.Oncoimmunology. 2015 Dec 8;5(2):e1117740. doi: 10.1080/2162402X.2015.1117740. eCollection 2016 Feb. Oncoimmunology. 2015. PMID: 27057469 Free PMC article. Review.
-
Autonomous parvoviruses neither stimulate nor are inhibited by the type I interferon response in human normal or cancer cells.J Virol. 2014 May;88(9):4932-42. doi: 10.1128/JVI.03508-13. Epub 2014 Feb 19. J Virol. 2014. PMID: 24554651 Free PMC article.
-
Trial Watch:: Oncolytic viruses for cancer therapy.Oncoimmunology. 2014 Jun 1;3:e28694. doi: 10.4161/onci.28694. eCollection 2014. Oncoimmunology. 2014. PMID: 25097804 Free PMC article. Review.
References
-
- Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM, Kirn DH, Freeman SM. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9(2):555–561. - PubMed
-
- Gordon EM, Chan MT, Geraldino N, Lopez FF, Cornelio GH, Lorenzo CC, 3rd, Levy JP, Reed RA, Liu L, Hall FL. Le morte du tumour: histological features of tumor destruction in chemo-resistant cancers following intravenous infusions of pathotropic nanoparticles bearing therapeutic genes. Int J Oncol. 2007;30(6):1297–1307. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

