Blood-brain barrier integrity and glial support: mechanisms that can be targeted for novel therapeutic approaches in stroke
- PMID: 22574987
- PMCID: PMC3918413
- DOI: 10.2174/138161212802002625
Blood-brain barrier integrity and glial support: mechanisms that can be targeted for novel therapeutic approaches in stroke
Abstract
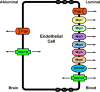
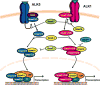
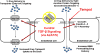
The blood-brain barrier (BBB) is a critical regulator of brain homeostasis. Additionally, the BBB is the most significant obstacle to effective CNS drug delivery. It possesses specific charcteristics (i.e., tight junction protein complexes, influx and efflux transporters) that control permeation of circulating solutes including therapeutic agents. In order to form this "barrier," brain microvascular endothelial cells require support of adjacent astrocytes and microglia. This intricate relationship also occurs between endothelial cells and other cell types and structures of the CNS (i.e., pericytes, neurons, extracellular matrix), which implies existence of a "neurovascular unit." Ischemic stroke can disrupt the neurovascular unit at both the structural and functional level, which leads to an increase in leak across the BBB. Recent studies have identified several pathophysiological mechanisms (i.e., oxidative stress, activation of cytokine-mediated intracellular signaling systems) that mediate changes in the neurovascular unit during ischemic stroke. This review summarizes current knowledge in this area and emphasizes pathways (i.e., oxidative stress, cytokine-mediated intracellular signaling, glial-expressed receptors/targets) that can be manipulated pharmacologically for i) preservation of BBB and glial integrity during ischemic stroke and ii) control of drug permeation and/or transport across the BBB. Targeting these pathways present a novel opportunity for optimization of CNS delivery of therapeutics in the setting of ischemic stroke.
Conflict of interest statement
Part of the information that appears in this article has been adapted from Ronaldson & Davis.
Figures
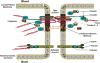

Similar articles
-
Regulation of blood-brain barrier integrity by microglia in health and disease: A therapeutic opportunity.J Cereb Blood Flow Metab. 2020 Dec;40(1_suppl):S6-S24. doi: 10.1177/0271678X20951995. Epub 2020 Sep 14. J Cereb Blood Flow Metab. 2020. PMID: 32928017 Free PMC article. Review.
-
Targeting transporters: promoting blood-brain barrier repair in response to oxidative stress injury.Brain Res. 2015 Oct 14;1623:39-52. doi: 10.1016/j.brainres.2015.03.018. Epub 2015 Mar 18. Brain Res. 2015. PMID: 25796436 Free PMC article. Review.
-
Crosstalk Among Glial Cells in the Blood-Brain Barrier Injury After Ischemic Stroke.Mol Neurobiol. 2024 Sep;61(9):6161-6174. doi: 10.1007/s12035-024-03939-6. Epub 2024 Jan 27. Mol Neurobiol. 2024. PMID: 38279077 Review.
-
Blood-brain barrier dysfunction in ischemic stroke: targeting tight junctions and transporters for vascular protection.Am J Physiol Cell Physiol. 2018 Sep 1;315(3):C343-C356. doi: 10.1152/ajpcell.00095.2018. Epub 2018 Jun 27. Am J Physiol Cell Physiol. 2018. PMID: 29949404 Free PMC article. Review.
-
Targeting blood-brain barrier changes during inflammatory pain: an opportunity for optimizing CNS drug delivery.Ther Deliv. 2011 Aug;2(8):1015-41. doi: 10.4155/tde.11.67. Ther Deliv. 2011. PMID: 22468221 Free PMC article. Review.
Cited by
-
Ischemic Preconditioning Alleviates Cerebral Ischemia-Reperfusion Injury by Interfering With Glycocalyx.Transl Stroke Res. 2023 Dec;14(6):929-940. doi: 10.1007/s12975-022-01081-w. Epub 2022 Sep 28. Transl Stroke Res. 2023. PMID: 36168082
-
Targeted drug delivery to treat pain and cerebral hypoxia.Pharmacol Rev. 2013 Jan 23;65(1):291-314. doi: 10.1124/pr.112.005991. Print 2013 Jan. Pharmacol Rev. 2013. PMID: 23343976 Free PMC article. Review.
-
Ischemic neurons activate astrocytes to disrupt endothelial barrier via increasing VEGF expression.J Neurochem. 2014 Apr;129(1):120-9. doi: 10.1111/jnc.12611. Epub 2013 Dec 6. J Neurochem. 2014. PMID: 24251624 Free PMC article.
-
CLEC14A deficiency exacerbates neuronal loss by increasing blood-brain barrier permeability and inflammation.J Neuroinflammation. 2020 Feb 4;17(1):48. doi: 10.1186/s12974-020-1727-6. J Neuroinflammation. 2020. PMID: 32019570 Free PMC article.
-
Downregulation of S1P Lyase Improves Barrier Function in Human Cerebral Microvascular Endothelial Cells Following an Inflammatory Challenge.Int J Mol Sci. 2020 Feb 13;21(4):1240. doi: 10.3390/ijms21041240. Int J Mol Sci. 2020. PMID: 32069843 Free PMC article.
References
-
- Hartz AM, Bauer B. Regulation of ABC transporters at the blood-brain barrier: new targets for CNS therapy. Mol Interv. 2010;10(5):293–304. - PubMed
-
- Witt KA, Mark KS, Hom S, Davis TP. Effects of hypoxia-reoxygenation on rat blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol. 2003;285(6):H2820–H2831. - PubMed
-
- Brown RC, Mark KS, Egleton RD, Davis TP. Protection against hypoxia-induced blood-brain barrier disruption: changes in intracellular calcium. Am J Physiol Cell Physiol. 2004;286(5):C1045–C1052. - PubMed
-
- Mark KS, Burroughs AR, Brown RC, Huber JD, Davis TP. Nitric oxide mediates hypoxia-induced changes in paracellular permeability of cerebral microvasculature. Am J Physiol Heart Circ Physiol. 2004;286(1):H174–H180. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

