Disruption of nongenomic testosterone signaling in a model of spinal and bulbar muscular atrophy
- PMID: 22570336
- PMCID: PMC5416995
- DOI: 10.1210/me.2011-1367
Disruption of nongenomic testosterone signaling in a model of spinal and bulbar muscular atrophy
Abstract
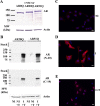
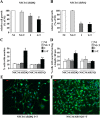
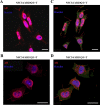
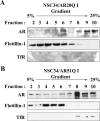
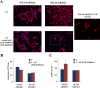
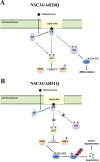
As one of the nine hereditary neurodegenerative polyQ disorders, spinal and bulbar muscular atrophy (SBMA) results from a polyQ tract expansion in androgen receptor (AR). Although protein aggregates are the pathological hallmark of many neurodegenerative diseases, their direct role in the neurodegeneration is more and more questioned. To determine the early molecular mechanisms causing motor neuron degeneration in SBMA, we established an in vitro system based on the tetracycline-inducible expression of normal (AR20Q), the mutated, 51 glutamine-extended (AR51Q), or polyQ-deleted (AR0Q) AR in NSC34, a motor neuron-like cell line lacking endogenous AR. Although no intracellular aggregates were formed, the expression of the AR51Q leads to a loss of function characterized by reduced neurite outgrowth and to a toxic gain of function resulting in decreased cell viability. In this study, we show that both AR20Q and AR51Q are recruited to lipid rafts in response to testosterone stimulation. However, whereas testosterone induces the activation of the c-jun N-terminal kinase/c-jun pathway via membrane-associated AR20Q, it does not so in NSC34 expressing AR51Q. Phosphorylation of c-jun N-terminal kinase plays a crucial role in AR20Q-dependent survival and differentiation of NSC34. Moreover, c-jun protein levels decrease more slowly in AR20Q- than in AR51Q-expressing NSC34 cells. This is due to a rapid and transient inhibition of glycogen synthase kinase 3α occurring in a phosphatidylinositol 3-kinase-independent manner. Our results demonstrate that the deregulation of nongenomic AR signaling may be involved in SBMA establishment, opening new therapeutic perspectives.
Figures




Similar articles
-
Polyglutamine-expanded androgen receptor truncation fragments activate a Bax-dependent apoptotic cascade mediated by DP5/Hrk.J Neurosci. 2009 Feb 18;29(7):1987-97. doi: 10.1523/JNEUROSCI.4072-08.2009. J Neurosci. 2009. PMID: 19228953 Free PMC article.
-
Loss of endogenous androgen receptor protein accelerates motor neuron degeneration and accentuates androgen insensitivity in a mouse model of X-linked spinal and bulbar muscular atrophy.Hum Mol Genet. 2006 Jul 15;15(14):2225-38. doi: 10.1093/hmg/ddl148. Epub 2006 Jun 13. Hum Mol Genet. 2006. PMID: 16772330
-
Adenylyl cyclase activating polypeptide reduces phosphorylation and toxicity of the polyglutamine-expanded androgen receptor in spinobulbar muscular atrophy.Sci Transl Med. 2016 Dec 21;8(370):370ra181. doi: 10.1126/scitranslmed.aaf9526. Sci Transl Med. 2016. PMID: 28003546 Free PMC article.
-
Polyglutamine tract expansion of the androgen receptor in a motoneuronal model of spinal and bulbar muscular atrophy.Brain Res Bull. 2001 Oct-Nov 1;56(3-4):215-20. doi: 10.1016/s0361-9230(01)00652-9. Brain Res Bull. 2001. PMID: 11719253 Review.
-
Pathogenesis and therapy of spinal and bulbar muscular atrophy (SBMA).Prog Neurobiol. 2012 Dec;99(3):246-56. doi: 10.1016/j.pneurobio.2012.05.007. Epub 2012 May 15. Prog Neurobiol. 2012. PMID: 22609045 Review.
Cited by
-
Oxidative Stress in DNA Repeat Expansion Disorders: A Focus on NRF2 Signaling Involvement.Biomolecules. 2020 May 1;10(5):702. doi: 10.3390/biom10050702. Biomolecules. 2020. PMID: 32369911 Free PMC article. Review.
-
Mechanisms mediating spinal and bulbar muscular atrophy: investigations into polyglutamine-expanded androgen receptor function and dysfunction.Front Neurol. 2013 May 15;4:53. doi: 10.3389/fneur.2013.00053. eCollection 2013. Front Neurol. 2013. PMID: 23720649 Free PMC article.
-
Sex and dose-dependent antinociceptive effects of the JNK (c-Jun N-terminal kinase) inhibitor SU 3327 are mediated by CB2 receptors in female, and CB1/CB2 receptors in male mice in an inflammatory pain model.Brain Res Bull. 2021 Dec;177:39-52. doi: 10.1016/j.brainresbull.2021.09.004. Epub 2021 Sep 14. Brain Res Bull. 2021. PMID: 34530070 Free PMC article.
-
The polyglutamine-expanded androgen receptor responsible for spinal and bulbar muscular atrophy inhibits the APC/C(Cdh1) ubiquitin ligase complex.Sci Rep. 2016 Jun 17;6:27703. doi: 10.1038/srep27703. Sci Rep. 2016. PMID: 27312068 Free PMC article.
-
Mutant androgen receptor induces neurite loss and senescence independently of ARE binding in a neuronal model of SBMA.Proc Natl Acad Sci U S A. 2024 Jul 16;121(29):e2321408121. doi: 10.1073/pnas.2321408121. Epub 2024 Jul 8. Proc Natl Acad Sci U S A. 2024. PMID: 38976730 Free PMC article.
References
-
- Zoghbi HY , Orr HT. 2000. Glutamine repeats and neurodegeneration. Annu Rev Neurosci 23:217–247 - PubMed
-
- Kennedy WR , Alter M , Sung JH. 1968. Progressive proximal spinal and bulbar muscular atrophy of late onset. A sex-linked recessive trait. Neurology 18:671–680 - PubMed
-
- La Spada AR , Wilson EM , Lubahn DB , Harding AE , Fischbeck KH. 1991. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 352:77–79 - PubMed
-
- Li M , Miwa S , Kobayashi Y , Merry DE , Yamamoto M , Tanaka F , Doyu M , Hashizume Y , Fischbeck KH , Sobue G. 1998. Nuclear inclusions of the androgen receptor protein in spinal and bulbar muscular atrophy. Ann Neurol 44:249–254 - PubMed
-
- McCampbell A , Taylor JP , Taye AA , Robitschek J , Li M , Walcott J , Merry D , Chai Y , Paulson H , Sobue G , Fischbeck KH. 2000. CREB-binding protein sequestration by expanded polyglutamine. Hum Mol Genet 9:2197–2202 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

