MFG-E8 released by apoptotic endothelial cells triggers anti-inflammatory macrophage reprogramming
- PMID: 22558449
- PMCID: PMC3340380
- DOI: 10.1371/journal.pone.0036368
MFG-E8 released by apoptotic endothelial cells triggers anti-inflammatory macrophage reprogramming
Abstract
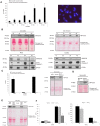
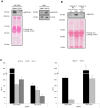
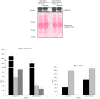
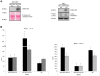
Apoptotic endothelial cells are an important component of the "response to injury" process. Several atherosclerosis risk factors such as hyperglycemia and oxidized low-density lipoproteins, and immune injuries, such as antibodies and complement, induce endothelial cell apoptosis. While endothelial cell apoptosis is known to affect neighboring vascular wall cell biology, its consequences on macrophage reprogramming are ill defined. In this study, we report that apoptosis of human and mouse endothelial cells triggers the release of milk fat globule-epidermal growth factor 8 (MFG-E8) and reprograms macrophages into an anti-inflammatory cells. We demonstrated that MFG-E8 is released by apoptotic endothelial cells in a caspase-3-dependent manner. When macrophages were exposed to conditioned media from serum-starved apoptotic endothelial cells, they adopt a high anti-inflammatory, low pro-inflammatory cytokine/chemokine secreting phenotype that is lost if MFG-E8 is absent from the media. Macrophage treatment with recombinant MFG-E8 recapitulates the effect of conditioned media. Finally, we showed that MFG-E8-mediated reprogramming of macrophages occurs through increased phosphorylation of signal transducer and activator of transcription-3 (STAT-3). Taken together, our study suggests a key role of MFG-E8 release from apoptotic endothelial cells in macrophage reprogramming and demonstrates the importance of the apoptotic microenvironment in anti-inflammatory macrophage responses.
Conflict of interest statement
Figures

Similar articles
-
Milk fat globule epidermal growth factor-8 limits tissue damage through inflammasome modulation during renal injury.J Leukoc Biol. 2016 Nov;100(5):1135-1146. doi: 10.1189/jlb.3A0515-213RR. Epub 2016 Jun 3. J Leukoc Biol. 2016. PMID: 27260955
-
Correction of MFG-E8 Resolves Inflammation and Promotes Cutaneous Wound Healing in Diabetes.J Immunol. 2016 Jun 15;196(12):5089-100. doi: 10.4049/jimmunol.1502270. Epub 2016 May 18. J Immunol. 2016. PMID: 27194784 Free PMC article.
-
MFG-E8 Reduces Aortic Intimal Proliferation in a Murine Model of Transplant Vasculopathy.Int J Mol Sci. 2022 Apr 7;23(8):4094. doi: 10.3390/ijms23084094. Int J Mol Sci. 2022. PMID: 35456911 Free PMC article.
-
Review: milk fat globule-EGF factor 8 expression, function and plausible signal transduction in resolving inflammation.Apoptosis. 2011 Nov;16(11):1077-86. doi: 10.1007/s10495-011-0630-0. Apoptosis. 2011. PMID: 21901532 Review.
-
Milk fat globule epidermal growth factor VIII signaling in arterial wall remodeling.Curr Vasc Pharmacol. 2013 Sep;11(5):768-76. doi: 10.2174/1570161111311050014. Curr Vasc Pharmacol. 2013. PMID: 22272902 Free PMC article. Review.
Cited by
-
MFGE8 in exosomes derived from mesenchymal stem cells prevents esophageal stricture after endoscopic submucosal dissection in pigs.J Nanobiotechnology. 2024 Apr 1;22(1):143. doi: 10.1186/s12951-024-02429-0. J Nanobiotechnology. 2024. PMID: 38561800 Free PMC article.
-
Milk Fat Globule-Epidermal Growth Factor 8 (MFG-E8) Is a Novel Anti-inflammatory Factor in Rheumatoid Arthritis in Mice and Humans.J Bone Miner Res. 2016 Mar;31(3):596-605. doi: 10.1002/jbmr.2721. Epub 2015 Nov 9. J Bone Miner Res. 2016. PMID: 26391522 Free PMC article.
-
Phagocytic clearance of apoptotic, necrotic, necroptotic and pyroptotic cells.Biochem Soc Trans. 2021 Apr 30;49(2):793-804. doi: 10.1042/BST20200696. Biochem Soc Trans. 2021. PMID: 33843978 Free PMC article. Review.
-
MFG-E8 mediates arterial aging by promoting the proinflammatory phenotype of vascular smooth muscle cells.J Biomed Sci. 2019 Aug 30;26(1):61. doi: 10.1186/s12929-019-0559-0. J Biomed Sci. 2019. PMID: 31470852 Free PMC article.
-
Circulating milk fat globule-epidermal growth factor 8 levels are increased in pregnancy and gestational diabetes mellitus.J Diabetes Investig. 2017 Jul;8(4):571-581. doi: 10.1111/jdi.12616. Epub 2017 Feb 15. J Diabetes Investig. 2017. PMID: 28035763 Free PMC article.
References
-
- Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. - PubMed
-
- Schaub FJ, Han DK, Liles WC, Adams LD, Coats SA, et al. Fas/FADD-mediated activation of a specific program of inflammatory gene expression in vascular smooth muscle cells. Nat Med. 2000;6:790–796. - PubMed
-
- Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–730. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

