A small molecule SMAC mimic LBW242 potentiates TRAIL- and anticancer drug-mediated cell death of ovarian cancer cells
- PMID: 22558117
- PMCID: PMC3338831
- DOI: 10.1371/journal.pone.0035073
A small molecule SMAC mimic LBW242 potentiates TRAIL- and anticancer drug-mediated cell death of ovarian cancer cells
Abstract
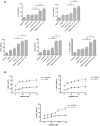
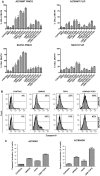
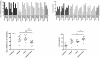
Background: Ovarian cancer remains a leading cause of death in women and development of new therapies is essential. Second mitochondria derived activator of caspase (SMAC) has been described to sensitize for apoptosis. We have explored the pro-apoptotic activity of LBW242, a mimic of SMAC/DIABLO, on ovarian cancer cell lines (A2780 cells and its chemoresistant derivative A2780/ADR, SKOV3 and HEY cells) and in primary ovarian cancer cells. The effects of LBW242 on ovarian cancer cell lines and primary ovarian cancer cells was determined by cell proliferation, apoptosis and biochemical assays.
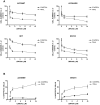
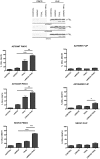
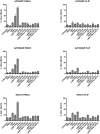
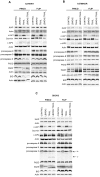
Principal findings: LBW242 added alone elicited only a moderate pro-apoptotic effect; however, it strongly synergizes with tumor necrosis factor-related apoptosis inducing ligand (TRAIL) or anticancer drugs in inducing apoptosis of both ovarian cancer cell lines and primary ovarian cancer cells. Mechanistic studies show that LBW242-induced apoptosis in ovarian cancer cells is associated with activation of caspase-8. In line with this mechanism, c-FLIP overexpression inhibits LBW242-mediated apoptosis.
Conclusion: LBW242 sensitizes ovarian cancer cells to the antitumor effects of TRAIL and anticancer drugs commonly used in clinic. These observations suggest that the SMAC/DIABLO mimic LBW242 could be of value for the development of experimental strategies for treatment of ovarian cancer.
Conflict of interest statement
Figures
Similar articles
-
A small molecule Smac mimic potentiates TRAIL-mediated cell death of ovarian cancer cells.Gynecol Oncol. 2007 May;105(2):481-92. doi: 10.1016/j.ygyno.2007.01.011. Epub 2007 Feb 9. Gynecol Oncol. 2007. PMID: 17292950
-
Smac peptide potentiates TRAIL- or paclitaxel-mediated ovarian cancer cell death in vitro and in vivo.Oncol Rep. 2013 Feb;29(2):515-22. doi: 10.3892/or.2012.2132. Epub 2012 Nov 9. Oncol Rep. 2013. PMID: 23151974
-
Smac/DIABLO enhances the therapeutic potential of chemotherapeutic drugs and irradiation, and sensitizes TRAIL-resistant breast cancer cells.Mol Cancer. 2008 Jun 30;7:60. doi: 10.1186/1476-4598-7-60. Mol Cancer. 2008. PMID: 18590557 Free PMC article.
-
Proteasome inhibitors sensitize ovarian cancer cells to TRAIL induced apoptosis.Apoptosis. 2007 Apr;12(4):635-55. doi: 10.1007/s10495-006-0025-9. Apoptosis. 2007. PMID: 17252198
-
The role of the mitochondria in mediating cytotoxicity of anti-cancer therapies.J Bioenerg Biomembr. 2007 Feb;39(1):13-21. doi: 10.1007/s10863-006-9055-9. J Bioenerg Biomembr. 2007. PMID: 17294132 Review.
Cited by
-
HRD Testing of Ovarian Cancer in Routine Practice: What Are We Dealing With?Int J Mol Sci. 2023 Jun 22;24(13):10497. doi: 10.3390/ijms241310497. Int J Mol Sci. 2023. PMID: 37445679 Free PMC article.
-
Direct estimation of differential networks.Biometrika. 2014 Jun;101(2):253-268. doi: 10.1093/biomet/asu009. Biometrika. 2014. PMID: 26023240 Free PMC article.
-
TRAIL-R2-specific antibodies and recombinant TRAIL can synergise to kill cancer cells.Oncogene. 2015 Apr 16;34(16):2138-2144. doi: 10.1038/onc.2014.156. Epub 2014 Jun 9. Oncogene. 2015. PMID: 24909167 Free PMC article.
-
Potency and Selectivity of SMAC/DIABLO Mimetics in Solid Tumor Therapy.Cells. 2020 Apr 18;9(4):1012. doi: 10.3390/cells9041012. Cells. 2020. PMID: 32325691 Free PMC article. Review.
-
Sigma-2 receptor ligand as a novel method for delivering a SMAC mimetic drug for treating ovarian cancer.Br J Cancer. 2013 Oct 29;109(9):2368-77. doi: 10.1038/bjc.2013.593. Epub 2013 Oct 8. Br J Cancer. 2013. PMID: 24104966 Free PMC article.
References
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Vucic D. Targeting IAP (inhibitor of apoptosis) proteins for therapeutic intervention in tumors. Curr Cancer Drug Targets. 2008;8:110–117. - PubMed
-
- La Casse EC, Mahoney DJ, Cheung HH, Plenchette S, Baird S, et al. IAP-targeted therapies for cancer. Oncogene. 2008;27:6252–6275. - PubMed
-
- Gyrd-Hansen M, Meier P. IAPs: from caspase inhibitors to modulators of NF-κB, inflammation and cancer. Nat Rev Cancer. 2010;10:561–574. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

