Loss of TMF/ARA160 protein renders colonic mucus refractory to bacterial colonization and diminishes intestinal susceptibility to acute colitis
- PMID: 22553199
- PMCID: PMC3408173
- DOI: 10.1074/jbc.M112.364786
Loss of TMF/ARA160 protein renders colonic mucus refractory to bacterial colonization and diminishes intestinal susceptibility to acute colitis
Abstract
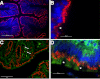
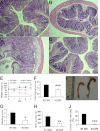
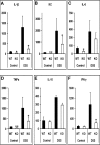
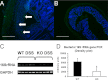
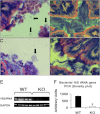
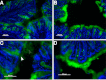
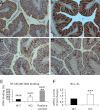
TMF/ARA160 is a Golgi-associated protein with several cellular functions, among them direction of the NF-κB subunit, p65 RelA, to ubiquitination and proteasomal degradation in stressed cells. We sought to investigate the role of TMF/ARA160 under imposed stress conditions in vivo. TMF(-/-) and wild-type (WT) mice were treated with the ulcerative agent dextran sulfate sodium (DSS), and the severity of the inflicted acute colitis was determined. TMF(-/-) mice were found to be significantly less susceptible to DSS-induced colitis, with profoundly less bacterial penetration into the colonic epithelia. Surprisingly, unlike in WT mice, no bacterial colonies were visualized in colons of healthy untreated TMF(-/-) mice, indicating the constitutive resistance of TMF(-/-) colonic mucus to bacterial retention and penetration. Gene expression analysis of colon tissues from unchallenged TMF(-/-) mice revealed 5-fold elevated transcription of the muc2 gene, which encodes the major component of the colonic mucus gel, the MUC2 mucin. Accordingly, the morphology of the colonic mucus in TMF(-/-) mice was found to differ from the mucus structure in WT colons. The NF-κB subunit, p65, a well known transcription inducer of muc2, was up-regulated significantly in TMF(-/-) intestinal epithelial cells. However, this did not cause spontaneous inflammation or increased colonic crypt cell proliferation. Collectively, our findings demonstrate that absence of TMF/ARA160 renders the colonic mucus refractory to bacterial colonization and the large intestine less susceptible to the onset of colitis.
Figures

Similar articles
-
Reprogrammed and transmissible intestinal microbiota confer diminished susceptibility to induced colitis in TMF-/- mice.Proc Natl Acad Sci U S A. 2014 Apr 1;111(13):4964-9. doi: 10.1073/pnas.1319114111. Epub 2014 Mar 17. Proc Natl Acad Sci U S A. 2014. PMID: 24639530 Free PMC article.
-
Importance and regulation of the colonic mucus barrier in a mouse model of colitis.Am J Physiol Gastrointest Liver Physiol. 2011 Feb;300(2):G327-33. doi: 10.1152/ajpgi.00422.2010. Epub 2010 Nov 25. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21109593 Free PMC article.
-
Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa.PLoS Pathog. 2010 May 13;6(5):e1000902. doi: 10.1371/journal.ppat.1000902. PLoS Pathog. 2010. PMID: 20485566 Free PMC article.
-
Muc2 Mucin and Nonmucin Microbiota Confer Distinct Innate Host Defense in Disease Susceptibility and Colonic Injury.Cell Mol Gastroenterol Hepatol. 2021;11(1):77-98. doi: 10.1016/j.jcmgh.2020.07.003. Epub 2020 Jul 10. Cell Mol Gastroenterol Hepatol. 2021. PMID: 32659381 Free PMC article.
-
MUC2 and related bacterial factors: Therapeutic targets for ulcerative colitis.EBioMedicine. 2021 Dec;74:103751. doi: 10.1016/j.ebiom.2021.103751. Epub 2021 Dec 10. EBioMedicine. 2021. PMID: 34902790 Free PMC article. Review.
Cited by
-
Sperm Differentiation: The Role of Trafficking of Proteins.Int J Mol Sci. 2020 May 24;21(10):3702. doi: 10.3390/ijms21103702. Int J Mol Sci. 2020. PMID: 32456358 Free PMC article. Review.
-
Activation of GABAA Receptors in Colon Epithelium Exacerbates Acute Colitis.Front Immunol. 2018 May 7;9:987. doi: 10.3389/fimmu.2018.00987. eCollection 2018. Front Immunol. 2018. PMID: 29867964 Free PMC article.
-
4-Octyl itaconate alleviates dextran sulfate sodium-induced ulcerative colitis in mice via activating the KEAP1-NRF2 pathway.Inflammopharmacology. 2024 Aug;32(4):2555-2574. doi: 10.1007/s10787-024-01490-3. Epub 2024 May 20. Inflammopharmacology. 2024. PMID: 38767761
-
The Physiological Functions of the Golgin Vesicle Tethering Proteins.Front Cell Dev Biol. 2019 Jun 18;7:94. doi: 10.3389/fcell.2019.00094. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31316978 Free PMC article. Review.
-
Delivery of a mucin domain enriched in cysteine residues strengthens the intestinal mucous barrier.Sci Rep. 2015 May 14;5:9577. doi: 10.1038/srep09577. Sci Rep. 2015. PMID: 25974250 Free PMC article.
References
-
- Yamane J., Kubo A., Nakayama K., Yuba-Kubo A., Katsuno T., Tsukita S., Tsukita S. (2007) Functional involvement of TMF/ARA160 in Rab6-dependent retrograde membrane traffic. Exp. Cell Res. 313, 3472–3485 - PubMed
-
- Abrham G., Volpe M., Shpungin S., Nir U. (2009) TMF/ARA160 down-regulates proangiogenic genes and attenuates the progression of PC3 xenografts. Int. J. Cancer 125, 43–53 - PubMed
-
- Perry E., Tsruya R., Levitsky P., Pomp O., Taller M., Weisberg S., Parris W., Kulkarni S., Malovani H., Pawson T., Shpungin S., Nir U. (2004) TMF/ARA160 is a BC-box-containing protein that mediates the degradation of Stat3. Oncogene 23, 8908–8919 - PubMed
-
- Lerer-Goldshtein T., Bel S., Shpungin S., Pery E., Motro B., Goldstein R. S., Bar-Sheshet S. I., Breitbart H., Nir U. (2010) TMF/ARA160: A key regulator of sperm development. Dev. Biol. 348, 12–21 - PubMed
-
- Podolsky D. K. (2002) Inflammatory bowel disease. N. Engl. J. Med. 347, 417–429 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

