The role played by perivascular cells in kidney interstitial injury
- PMID: 22551886
- PMCID: PMC4407340
- DOI: 10.5414/cn107371
The role played by perivascular cells in kidney interstitial injury
Abstract
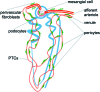
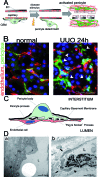
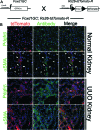
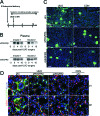
Fibrosis of the kidney is a disease affecting millions worldwide and is a harbinger of progressive loss of organ function resulting in organ failure. Recent findings suggest that understanding mechanisms of development and progression of fibrosis will lead to new therapies urgently required to counteract loss of organ function. Recently, little-known cells that line the kidney microvasculature, known as pericytes, were identified as the precursor cells which become the scar-forming myofibroblasts. Kidney pericytes are extensively branched cells located in the wall of capillaries, embedded within the microvascular basement membrane, and incompletely envelope endothelial cells with which they establish focal contacts. In response to kidney injuries, pericytes detach from endothelial cells and migrate into the interstitial space where they undergo a transition into myofibroblasts. Detachment leads to fibrosis but also leaves an unstable endothelium, prone to rarefaction. Endothelial-pericyte crosstalk at the vascular endothelial growth factor receptors and platelet derived growth factor receptors in response to injury have been identified as major new targets for therapeutic intervention.
Figures
Comment in
-
Pericytes and renal microvascular disease induce renal fibrosis in CKD.Clin Nephrol. 2013 Oct;80(4):310. doi: 10.5414/CN107797. Clin Nephrol. 2013. PMID: 24040780 No abstract available.
Similar articles
-
Novel insights into pericyte-myofibroblast transition and therapeutic targets in renal fibrosis.J Formos Med Assoc. 2012 Nov;111(11):589-98. doi: 10.1016/j.jfma.2012.09.008. Epub 2012 Oct 24. J Formos Med Assoc. 2012. PMID: 23217594 Review.
-
Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis.Am J Pathol. 2011 Feb;178(2):911-23. doi: 10.1016/j.ajpath.2010.10.012. Am J Pathol. 2011. PMID: 21281822 Free PMC article.
-
Pivotal role of pericytes in kidney fibrosis.Clin Exp Pharmacol Physiol. 2011 Jul;38(7):467-73. doi: 10.1111/j.1440-1681.2011.05531.x. Clin Exp Pharmacol Physiol. 2011. PMID: 21517936 Free PMC article. Review.
-
Endothelial sirtuin 1 inactivation enhances capillary rarefaction and fibrosis following kidney injury through Notch activation.Biochem Biophys Res Commun. 2016 Sep 23;478(3):1074-9. doi: 10.1016/j.bbrc.2016.08.066. Epub 2016 Aug 12. Biochem Biophys Res Commun. 2016. PMID: 27524235 Free PMC article.
-
Pericytes in kidney fibrosis.Curr Opin Nephrol Hypertens. 2013 Jul;22(4):471-80. doi: 10.1097/MNH.0b013e328362485e. Curr Opin Nephrol Hypertens. 2013. PMID: 23722183 Review.
Cited by
-
Ferroptosis, a Rising Force against Renal Fibrosis.Oxid Med Cell Longev. 2022 Oct 12;2022:7686956. doi: 10.1155/2022/7686956. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36275899 Free PMC article. Review.
-
The Yin and Yang of Mesenchymal Cells in the Corneal Stromal Fibrosis Response to Injury: The Cornea as a Model of Fibrosis in Other Organs.Biomolecules. 2022 Dec 31;13(1):87. doi: 10.3390/biom13010087. Biomolecules. 2022. PMID: 36671472 Free PMC article. Review.
-
What We Talk About When We Talk About Evolution.Cell Commun Insights. 2015;7:1-15. doi: 10.4137/CCI.S29840. Cell Commun Insights. 2015. PMID: 26702252 Free PMC article.
-
Sources of myofibroblasts in kidney fibrosis: all answers are correct, however to different extent!Int Urol Nephrol. 2014 Mar;46(3):659-64. doi: 10.1007/s11255-013-0626-5. Epub 2013 Dec 25. Int Urol Nephrol. 2014. PMID: 24368748
-
Deletion of Pkd1 in renal stromal cells causes defects in the renal stromal compartment and progressive cystogenesis in the kidney.Lab Invest. 2017 Dec;97(12):1427-1438. doi: 10.1038/labinvest.2017.97. Epub 2017 Sep 11. Lab Invest. 2017. PMID: 28892094
References
-
- Schrimpf C Duffield JS Mechanisms of fibrosis: the role of the pericyte. Curr Opin Nephrol Hypertens. 2011; 20: 297–305. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

