Efficacy and Safety of Duloxetine in Patients with Chronic Low Back Pain Who Used versus Did Not Use Concomitant Nonsteroidal Anti-Inflammatory Drugs or Acetaminophen: A Post Hoc Pooled Analysis of 2 Randomized, Placebo-Controlled Trials
- PMID: 22550577
- PMCID: PMC3324928
- DOI: 10.1155/2012/296710
Efficacy and Safety of Duloxetine in Patients with Chronic Low Back Pain Who Used versus Did Not Use Concomitant Nonsteroidal Anti-Inflammatory Drugs or Acetaminophen: A Post Hoc Pooled Analysis of 2 Randomized, Placebo-Controlled Trials
Abstract
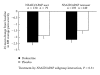
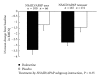
This subgroup analysis assessed the efficacy of duloxetine in patients with chronic low back pain (CLBP) who did or did not use concomitant nonsteroidal anti-inflammatory drugs (NSAIDs) or acetaminophen (APAP). Data were pooled from two 13-week randomized trials in patients with CLBP who were stratified according to NSAID/APAP use at baseline: duloxetine NSAID/APAP user (n = 137), placebo NSAID/APAP user (n = 82), duloxetine NSAID/APAP nonuser (n = 206), and placebo NSAID/APAP nonuser (n = 156). NSAID/APAP users were those patients who took NSAID/APAP for at least 14 days per month during 3 months prior to study entry. An analysis of covariance model that included therapy, study, baseline NSAID/APAP use (yes/no), and therapy-by-NSAID/APAP subgroup interaction was used to assess the efficacy. The treatment-by-NSAID/APAP use interaction was not statistically significant (P = 0.31) suggesting no substantial evidence of differential efficacy for duloxetine over placebo on pain reduction or improvement in physical function between concomitant NSAID/APAP users and non-users.
Figures

Similar articles
-
Efficacy of duloxetine by prior NSAID use in the treatment of chronic osteoarthritis knee pain: A post hoc subgroup analysis of a randomized, placebo-controlled, phase 3 study in Japan.J Orthop Sci. 2018 Nov;23(6):1019-1026. doi: 10.1016/j.jos.2018.07.008. Epub 2018 Aug 17. J Orthop Sci. 2018. PMID: 30126675 Clinical Trial.
-
The risk of bleeding with duloxetine treatment in patients who use nonsteroidal anti-inflammatory drugs (NSAIDs): analysis of placebo-controlled trials and post-marketing adverse event reports.Drug Healthc Patient Saf. 2013 Nov 25;5:211-9. doi: 10.2147/DHPS.S45445. eCollection 2013. Drug Healthc Patient Saf. 2013. PMID: 24348072 Free PMC article.
-
The efficacy of tramadol/acetaminophen combination tablets (Ultracet®) as add-on and maintenance therapy in knee osteoarthritis pain inadequately controlled by nonsteroidal anti-inflammatory drug (NSAID).Clin Rheumatol. 2012 Feb;31(2):317-23. doi: 10.1007/s10067-011-1818-y. Epub 2011 Aug 3. Clin Rheumatol. 2012. PMID: 21811797 Clinical Trial.
-
Oral non-steroidal anti-inflammatory drugs versus other oral analgesic agents for acute soft tissue injury.Cochrane Database Syst Rev. 2015 Jul 1;(7):CD007789. doi: 10.1002/14651858.CD007789.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2020 Aug 12;8:CD007789. doi: 10.1002/14651858.CD007789.pub3. PMID: 26130144 Updated. Review.
-
WITHDRAWN: Non-aspirin, non-steroidal anti-inflammatory drugs for treating osteoarthritis of the knee.Cochrane Database Syst Rev. 2007 Jul 18;2006(1):CD000142. doi: 10.1002/14651858.CD000142.pub2. Cochrane Database Syst Rev. 2007. PMID: 17636601 Free PMC article. Review.
Cited by
-
Non-steroidal anti-inflammatory drugs for sciatica.Cochrane Database Syst Rev. 2016 Oct 15;10(10):CD012382. doi: 10.1002/14651858.CD012382. Cochrane Database Syst Rev. 2016. PMID: 27743405 Free PMC article. Review.
-
Kynurenine and Tetrahydrobiopterin Pathways Crosstalk in Pain Hypersensitivity.Front Neurosci. 2020 Jun 24;14:620. doi: 10.3389/fnins.2020.00620. eCollection 2020. Front Neurosci. 2020. PMID: 32694973 Free PMC article. Review.
References
-
- Andersson GBJ. Epidemiological features of chronic low-back pain. The Lancet. 1999;354(9178):581–585. - PubMed
-
- Manchikanti L. Epidemiology of low back pain. Pain Physician. 2000;3(2):167–192. - PubMed
-
- Chang V, Gonzalez P, Akuthota V. Evidence-informed management of chronic low back pain with adjunctive analgesics. Spine Journal. 2008;8(1):21–27. - PubMed
-
- Chou R, Huffman LH. Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Annals of Internal Medicine. 2007;147(7):505–514. - PubMed
LinkOut - more resources
Full Text Sources
